
Concept explainers
Draw a structure corresponding to each name.
a.
b.
c.
d.
e.
f.
(a)
Interpretation: The structure which corresponds to the IUPAC name of the given compound is to be stated.
Concept introduction: IUPAC nomenclature is a systematic way of naming the organic compounds. The basic principles of IUPAC naming for benzene derivatives are:
1. The compound consists of benzene ring is named as substituent followed by benzene.
2. When the ring is mono-substituted, then there is no need of numbering.
3. When the ring is substituted with same substituents, then numbering to one substituent is given and for other substituent, numbering proceed from clockwise or anticlockwise such that it gets lower number.
4. When the ring is substituted with different substituents, then the numbering is done according to the priority.
5. Substituents on benzene ring is also indicated using ortho, meta, para prefix. The prefix ortho is used when substituents are on adjacent carbon, meta is used when substituents are separated by one carbon atom, para is used when substituents are across each other in benzene ring.
Answer to Problem 17.28P
The structure of the given compound is,
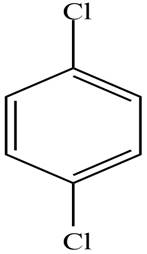
Explanation of Solution
The IUPAC name of the compound is
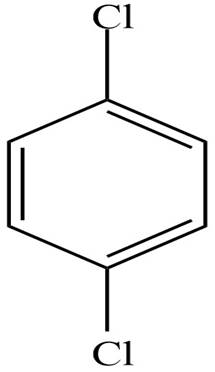
Figure 1
The structure of the given compound is shown in Figure 1.
(b)
Interpretation: The structure which corresponds to the IUPAC name of the given compound is to be stated.
Concept introduction: IUPAC nomenclature is a systematic way of naming the organic compounds. The basic principles of IUPAC naming for benzene derivatives are:
1. The compound consists of benzene ring is named as substituent followed by benzene.
2. When the ring is mono-substituted, then there is no need of numbering.
3. When the ring is substituted with same substituents, then numbering to one substituent is given and for other substituent, numbering proceed from clockwise or anticlockwise such that it gets lower number.
4. When the ring is substituted with different substituents, then the numbering is done according to the priority.
5. Substituents on benzene ring is also indicated using ortho, meta, para prefix. The prefix ortho is used when substituents are on adjacent carbon, meta is used when substituents are separated by one carbon atom, para is used when substituents are across each other in benzene ring.
Answer to Problem 17.28P
The structure of the given compound is,
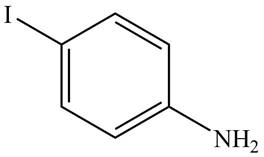
Explanation of Solution
The IUPAC name of the given compound is
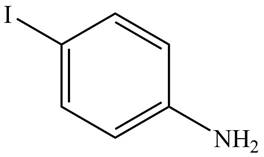
Figure 2
The structure of the given compound is shown in Figure 2.
(c)
Interpretation: The structure which corresponds to the IUPAC name of the given compound is to be stated.
Concept introduction: IUPAC nomenclature is a systematic way of naming the organic compounds. The basic principles of IUPAC naming for benzene derivatives are:
1. The compound consists of benzene ring is named as substituent followed by benzene.
2. When the ring is mono-substituted, then there is no need of numbering.
3. When the ring is substituted with same substituents, then numbering to one substituent is given and for other substituent, numbering proceed from clockwise or anticlockwise such that it gets lower number.
4. When the ring is substituted with different substituents, then the numbering is done according to the priority.
5. Substituents on benzene ring is also indicated using ortho, meta, para prefix. The prefix ortho is used when substituents are on adjacent carbon, meta is used when substituents are separated by one carbon atom, para is used when substituents are across each other in benzene ring.
Answer to Problem 17.28P
The structure of the given compound is,
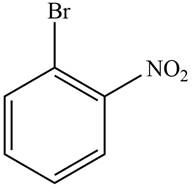
Explanation of Solution
The IUPAC name of the compound is

Figure 3
The structure of the given compound is shown in Figure 3.
(d)
Interpretation: The structure which corresponds to the IUPAC name of the given compound is to be stated.
Concept introduction: IUPAC nomenclature is a systematic way of naming the organic compounds. The basic principles of IUPAC naming for benzene derivatives are:
1. The compound consists of benzene ring is named as substituent followed by benzene.
2. When the ring is mono-substituted, then there is no need of numbering.
3. When the ring is substituted with same substituents, then numbering to one substituent is given and for other substituent, numbering proceed from clockwise or anticlockwise such that it gets lower number.
4. When the ring is substituted with different substituents, then the numbering is done according to the priority.
5. Substituents on benzene ring is also indicated using ortho, meta, para prefix. The prefix ortho is used when substituents are on adjacent carbon, meta is used when substituents are separated by one carbon atom, para is used when substituents are across each other in benzene ring.
Answer to Problem 17.28P
The structure of the given compound is,
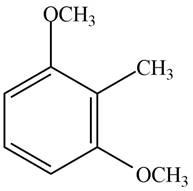
Explanation of Solution
The IUPAC name of the compound is
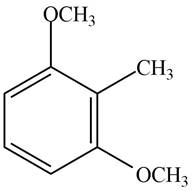
Figure 4
The structure of the given compound is shown in Figure 4.
(e)
Interpretation: The structure which corresponds to the IUPAC name of the given compound is to be stated.
Concept introduction: IUPAC nomenclature is a systematic way of naming the organic compounds. The basic principles of IUPAC naming for hydrocarbon are:
1. The hydrocarbon is named after the carbon chain containing higher number of carbon atoms.
2. Alkenes are named after their parent hydrocarbon chain with suffix ‘ene’.
3. For functional group such as alcohol suffix ‘ol’ is added to the name.
4. When the ring is substituted with different substituents, then the numbering is done according to the priority.
Answer to Problem 17.28P
The structure of the given compound is,
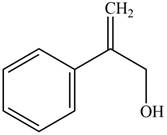
Explanation of Solution
The IUPAC name of the compound is
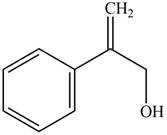
Figure 5
The structure of the given compound is shown in Figure 5.
(f)
Interpretation: The structure which corresponds to the IUPAC name of the given compound is to be stated.
Concept introduction: IUPAC nomenclature is a systematic way of naming the organic compounds. The basic principles of IUPAC naming for a cycloalkane are:
1. The cycloalkane is named after its parent hydrocarbon chain with a prefix cyclo.
2. When the ring is mono-substituted then there is no need of numbering.
3. When the ring is substituted with same substituents then numbering to one substituent is given and for other substituent numbering proceed from clockwise or anticlockwise such that it gets lower number.
4. When the ring is substituted with different substituents, then the numbering is done according to the priority.
5. When alkyl chain has more carbon atoms than the cycloalkane then, the substituent is named as cycloalkyl group.
Answer to Problem 17.28P
The structure of the given compound is,
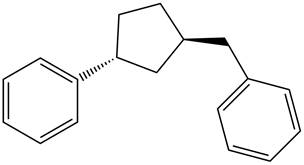
Explanation of Solution
The IUPAC name of the compound is
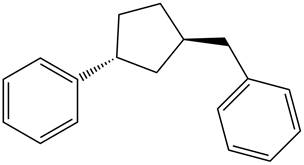
Figure 6
The structure of the given compound is shown in Figure 6.
Want to see more full solutions like this?
Chapter 17 Solutions
Package: Loose Leaf for Organic Chemistry with Biological Topics with Connect Access Card
- Don't used Ai solutionarrow_forward5. A solution of sucrose is fermented in a vessel until the evolution of CO2 ceases. Then, the product solution is analyzed and found to contain, 45% ethanol; 5% acetic acid; and 15% glycerin by weight. If the original charge is 500 kg, evaluate; e. The ratio of sucrose to water in the original charge (wt/wt). f. Moles of CO2 evolved. g. Maximum possible amount of ethanol that could be formed. h. Conversion efficiency. i. Per cent excess of excess reactant. Reactions: Inversion reaction: C12H22O11 + H2O →2C6H12O6 Fermentation reaction: C6H12O6 →→2C2H5OH + 2CO2 Formation of acetic acid and glycerin: C6H12O6 + C2H5OH + H₂O→ CH3COOH + 2C3H8O3arrow_forwardShow work. don't give Ai generated solution. How many carbons and hydrogens are in the structure?arrow_forward
- 13. (11pts total) Consider the arrows pointing at three different carbon-carbon bonds in the molecule depicted below. Bond B 2°C. +2°C. cleavage Bond A •CH3 + 26.← Cleavage 2°C. + Bond C +3°C• CH3 2C Cleavage E 2°C. 26. weakest bond Intact molecule Strongest 3°C 20. Gund Largest argest a. (2pts) Which bond between A-C is weakest? Which is strongest? Place answers in appropriate boxes. C Weakest bond A Produces Most Bond Strongest Bond Strongest Gund produces least stable radicals Weakest Stable radical b. (4pts) Consider the relative stability of all cleavage products that form when bonds A, B, AND C are homolytically cleaved/broken. Hint: cleavage products of bonds A, B, and C are all carbon radicals. i. Which ONE cleavage product is the most stable? A condensed or bond line representation is fine. 13°C. formed in bound C cleavage ii. Which ONE cleavage product is the least stable? A condensed or bond line representation is fine. • CH3 methyl radical Formed in Gund A Cleavage c.…arrow_forwardBr. COOH Br, FCH COOH E FeBr ASOCI B NH (CH,CO),OD Br₂ 2 C alcKOHarrow_forwardFind A to F (all)arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





