
(a)
Interpretation:
The preparation method of benzyl methyl ether from toluene is to be stated.
Concept introduction:
Cyclic
Answer to Problem 17.28AP
The preparation method of benzyl methyl ether from toluene is shown below.
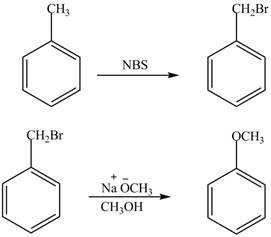
Explanation of Solution
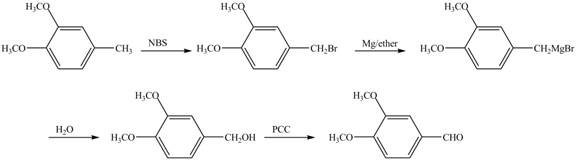
For preparing benzyl methyl ether from toluene, toluene is first brominated.
For bromination of toluene,
The corresponding reaction sequences are shown below.
Figure 1
The preparation method of benzyl methyl ether from toluene is shown in Figure 1.
(b)
Interpretation:
The preparation method of
Concept introduction:
Cyclic alkenes on reaction with N-Bromosuccinimide (NBS) forms allyl or benzyl bromide, that is, bromine is substituted at the allylic or benzylic position. NBS is a rich source of free radical of
Answer to Problem 17.28AP
The preparation method of
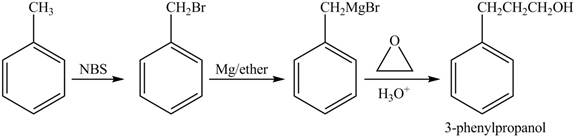
Explanation of Solution
In given reaction, toluene is first brominated with NBS (N-bromosuccinamide). Brominated benzyl reacts with Grignard reagent and
The corresponding reaction sequences to obtain the desired product are shown below.

Figure 2
The preparation method of
(c)
Interpretation:
The preparation method of
Concept introduction:
The Lindlar’s catalyst is composed of
Answer to Problem 17.28AP
The preparation method of
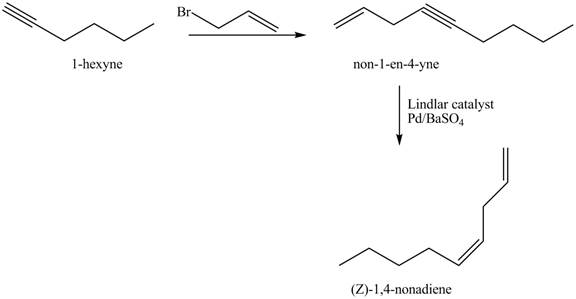
Explanation of Solution
The addition of
The corresponding reaction sequences to obtain the desired product are shown below.
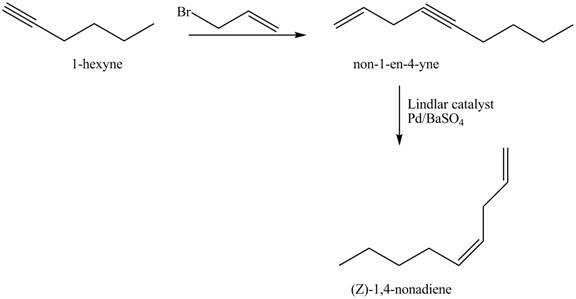
Figure 3
The preparation method of
(d)
Interpretation:
The preparation method of given compound from cyclopentene is to be stated.
Concept introduction:
Cyclic alkenes on reaction with N-Bromosuccinimide (NBS) forms allyl or benzyl bromide, that is, bromine is substituted at the allylic or benzylic position. NBS is a rich source of free radical of
Answer to Problem 17.28AP
The preparation method of given compound from cyclopentene is shown below.
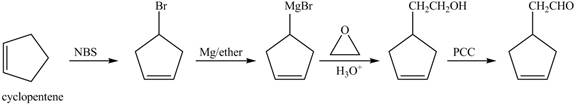
Explanation of Solution
Cyclopentene is first brominated and then on treatment with Grignard reagent and epoxide it gives corresponding alkyl alcohol. This alkyl alcohol is oxidized with mild reagent PCC to convert it into
The corresponding reaction sequences to obtain the desired product are shown below.
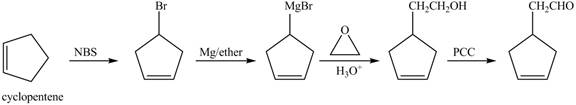
Figure 4
The preparation method of given compound from cyclopentene is shown in Figure 4.
(e)
Interpretation:
The preparation method of
Concept introduction:
The
Answer to Problem 17.28AP
The preparation method of
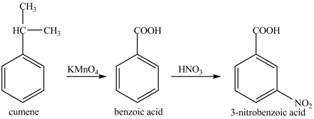
Explanation of Solution
Cumene has to be oxidized first and then its nitration is done to get the required product
Alkyl group is oxidized to gives benzoic acid. This benzoic acid is nitrated with
The corresponding reaction sequences to obtain the desired product are shown below.
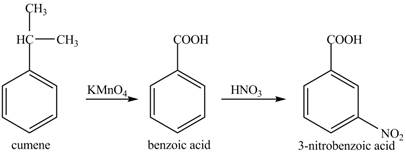
Figure 5
The preparation method of
(f)
Interpretation:
The preparation method of para-nitro benzoic acid from cumene (isopropyl benzene) is to be stated.
Concept introduction:
The chemical reaction in which an electrophile group is replaced by another functional group is known as the electrophilic substitution reaction. When the electrophilic substitution happens on an aromatic ring such as benzene then the reaction is known as electrophilic aromatic substitution.
Answer to Problem 17.28AP
The preparation method of para-nitro benzoic acid from cumene (isopropyl benzene) is shown below.
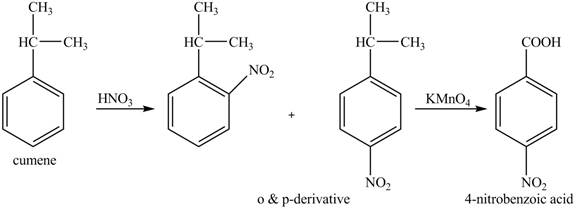
Explanation of Solution
As alkyl group is ortho and para directing, so, both the derivatives are formed. The reaction of cumene with nitric acid results in the formation of ortho and para nitro-cumene. The para nitro cumene is further oxidized to p-nitro benzoic acid with potassium permangnate.
The corresponding reaction sequences to obtain the desired product are shown below.
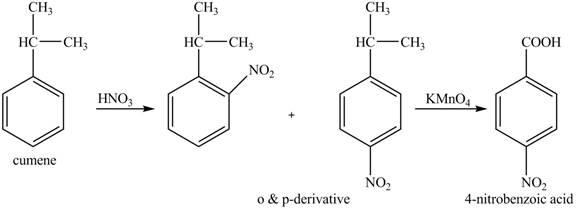
Figure 6
The preparation method of para-nitro benzoic acid from cumene (isopropyl benzene) is shown in Figure 6.
(g)
Interpretation:
The preparation method of
Concept introduction:
Cyclic alkenes on reaction with N-Bromosuccinimide (NBS) forms allyl or benzyl bromide, that is, bromine is substituted at the allylic or benzylic position. NBS is a rich source of free radical of
Answer to Problem 17.28AP
The preparation method of
Explanation of Solution
In the given reaction, at first, the substrate,
The corresponding reaction sequences to obtain the desired product are shown below.
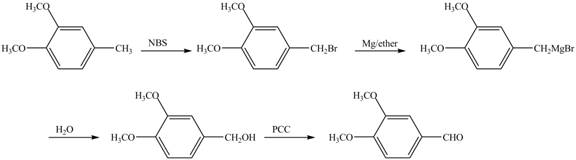
Figure 7
The preparation method of
(h)
Interpretation:
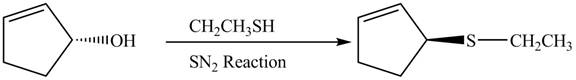
The preparation of given compound from cyclopentenol is to be given.
Concept introduction:
The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as a substitution reaction. In a nucleophilic substitution reaction, nucleophile takes the position of leaving the group by attacking the electron-deficient carbon atom.
Answer to Problem 17.28AP
The preparation of given compound from cyclopentenol is shown below.
Explanation of Solution
The given reaction is a nucleophilic substitution reaction. The nucleophile attacks to replace the
This is a single step substitution nucleophile reaction as shown below.
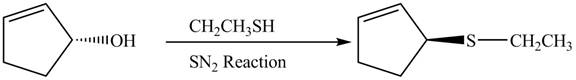
Figure 8
In this reaction, the stereochemistry of molecule changes as from one side the group is leaving and upon other side of the molecule, nucleophile attacks.
The preparation of given compound from cyclopentenol is shown in Figure 8.
Want to see more full solutions like this?
Chapter 17 Solutions
Organic Chemistry Study Guide and Solutions
- :0: :0: Select to Add Arrows :0: (CH3)2NH :0: ■ Select to Add Arrows :0: :0: (CH3)2NH ■ Select to Add Arrowsarrow_forwardDraw the product of the following H action sequence. Ignore any inorganic byproducts formed. 1. (CH3CH2)2CuLi, THF 2. CH3Br Q Atoms, Bonds and Rings H Charges ㅁarrow_forwardPlease help me with this the problem is so confusingarrow_forward
- 14 Question (1 point) Disiamylborane adds to a triple bond to give an alkenylborane. Upon oxidation with OH, H2O2, the alkenylborane will form an enol that tautomerizes to an aldehyde. In the first box below, draw the mechanism arrows for the reaction of disiamylborane with the alkyne, and in the last box draw the structure of the aldehyde. 4th attempt Feedback i > 3rd attempt OH, H2O2 i See Periodic Table See Hintarrow_forwardanswer with mechanisms and steps. handwritten please!arrow_forwardHello I need some help with Smartwork. For drawing structure B, I know the correct answer is CH₃B₂, but when I try to type it in, it keeps giving me CH₄BH₃ instead. Do you know how I should write it properly? Should I use a bond or something else?arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





