
(a)
Interpretation:
The preparation method of benzyl methyl ether from toluene is to be stated.
Concept introduction:
Cyclic
Answer to Problem 17.28AP
The preparation method of benzyl methyl ether from toluene is shown below.
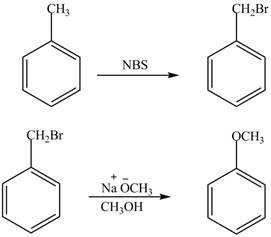
Explanation of Solution
For preparing benzyl methyl ether from toluene, toluene is first brominated.
For bromination of toluene, N− bromosuccinamide (NBS) is used in CCl4. Benzyl bromide on reaction with strong base gives the substituted ether.
The corresponding reaction sequences are shown below.
Figure 1
The preparation method of benzyl methyl ether from toluene is shown in Figure 1.
(b)
Interpretation:
The preparation method of 3−phenyl−1−propanol from toluene is to be stated.
Concept introduction:
Cyclic alkenes on reaction with N-Bromosuccinimide (NBS) forms allyl or benzyl bromide, that is, bromine is substituted at the allylic or benzylic position. NBS is a rich source of free radical of Br. The mechanism followed by NBS is a radical substitution reaction.
Answer to Problem 17.28AP
The preparation method of 3−phenyl−1−propanol from toluene is shown below.
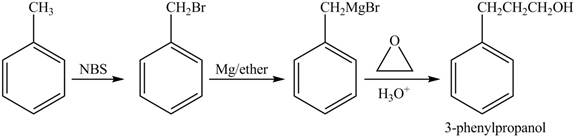
Explanation of Solution
In given reaction, toluene is first brominated with NBS (N-bromosuccinamide). Brominated benzyl reacts with Grignard reagent and
The corresponding reaction sequences to obtain the desired product are shown below.

Figure 2
The preparation method of 3−phenyl−1−propanol from toluene is shown in Figure 2.
(c)
Interpretation:
The preparation method of (Z)−1,4−nanodiene from 1−hexyne is to be stated.
Concept introduction:
The Lindlar’s catalyst is composed of H2 over platinum, traces of lead and quinoline. Lead and quinoline poisons the activity of this catalyst, so, that it reduces an alkyne into an alkene. The Lindlar’s catalyst is used to reduce the
Answer to Problem 17.28AP
The preparation method of (Z)−1,4−nanodiene from 1−hexyne is shown below.
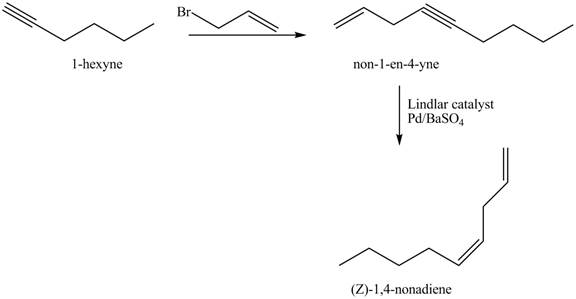
Explanation of Solution
The addition of 3−bromoprop−1−ene gives addition reaction with 1−hexyne. This product is further reduced to (Z)−1,4−nanodiene with the help of Lindlar catalyst.
The corresponding reaction sequences to obtain the desired product are shown below.
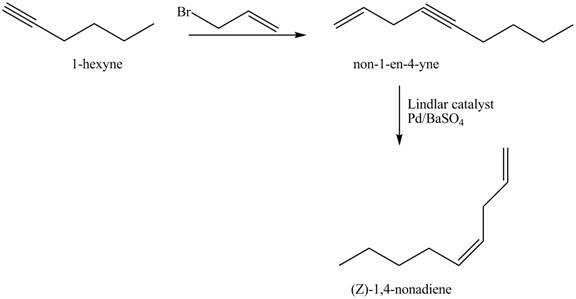
Figure 3
The preparation method of (Z)−1,4−nanodiene from 1−hexyne is shown in Figure 3.
(d)
Interpretation:
The preparation method of given compound from cyclopentene is to be stated.
Concept introduction:
Cyclic alkenes on reaction with N-Bromosuccinimide (NBS) forms allyl or benzyl bromide, that is, bromine is substituted at the allylic or benzylic position. NBS is a rich source of free radical of Br. The mechanism followed by NBS is a radical substitution reaction.
Answer to Problem 17.28AP
The preparation method of given compound from cyclopentene is shown below.
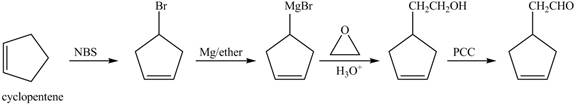
Explanation of Solution
Cyclopentene is first brominated and then on treatment with Grignard reagent and epoxide it gives corresponding alkyl alcohol. This alkyl alcohol is oxidized with mild reagent PCC to convert it into
The corresponding reaction sequences to obtain the desired product are shown below.
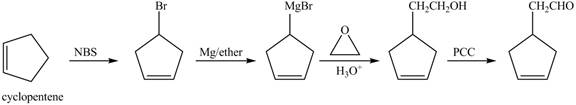
Figure 4
The preparation method of given compound from cyclopentene is shown in Figure 4.
(e)
Interpretation:
The preparation method of 3− nitro benzoic acid from cumene (isopropyl benzene) is to be stated.
Concept introduction:
The
Answer to Problem 17.28AP
The preparation method of 3− nitro benzoic acid from cumene (isopropyl benzene) is shown below.
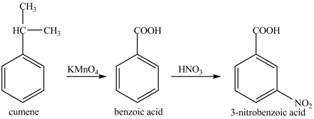
Explanation of Solution
Cumene has to be oxidized first and then its nitration is done to get the required product
Alkyl group is oxidized to gives benzoic acid. This benzoic acid is nitrated with HNO3 to obtain 3− nitro benzoic acid.
The corresponding reaction sequences to obtain the desired product are shown below.
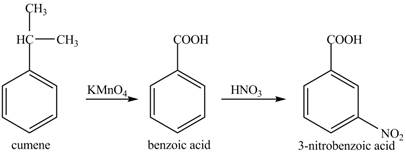
Figure 5
The preparation method of 3− nitro benzoic acid from cumene (isopropyl benzene) is shown in Figure 5.
(f)
Interpretation:
The preparation method of para-nitro benzoic acid from cumene (isopropyl benzene) is to be stated.
Concept introduction:
The chemical reaction in which an electrophile group is replaced by another functional group is known as the electrophilic substitution reaction. When the electrophilic substitution happens on an aromatic ring such as benzene then the reaction is known as electrophilic aromatic substitution.
Answer to Problem 17.28AP
The preparation method of para-nitro benzoic acid from cumene (isopropyl benzene) is shown below.
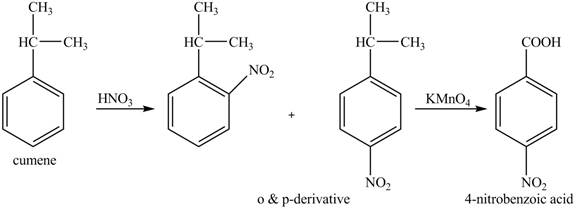
Explanation of Solution
As alkyl group is ortho and para directing, so, both the derivatives are formed. The reaction of cumene with nitric acid results in the formation of ortho and para nitro-cumene. The para nitro cumene is further oxidized to p-nitro benzoic acid with potassium permangnate.
The corresponding reaction sequences to obtain the desired product are shown below.
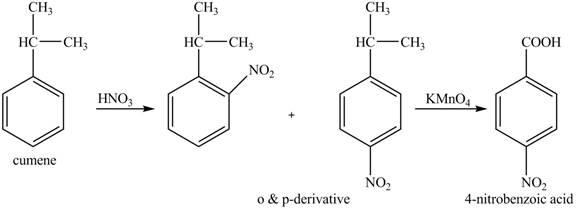
Figure 6
The preparation method of para-nitro benzoic acid from cumene (isopropyl benzene) is shown in Figure 6.
(g)
Interpretation:
The preparation method of 1,2−dimethoxy−4−benzaldehyde from 1,2−dimethoxy−4−methylbenzene is to be stated.
Concept introduction:
Cyclic alkenes on reaction with N-Bromosuccinimide (NBS) forms allyl or benzyl bromide, that is, bromine is substituted at the allylic or benzylic position. NBS is a rich source of free radical of Br. The mechanism followed by NBS is a radical substitution reaction.
Answer to Problem 17.28AP
The preparation method of 1,2−dimethoxy−4−benzaldehyde from 1,2−dimethoxy−4−methylbenzene is shown below.
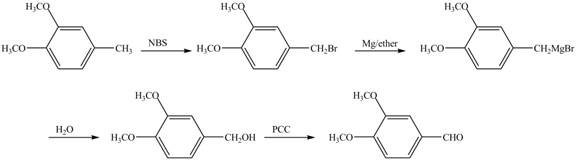
Explanation of Solution
In the given reaction, at first, the substrate, 1,2−dimethoxy−4−methylbenzene is brominated with NBS and then converted to Grignard reagent by the reaction of Mg in ether. This is further hydrolyzed to produce alcohol which is oxidized with mild oxidizing agent PCC to convert it to required aldehyde.
The corresponding reaction sequences to obtain the desired product are shown below.
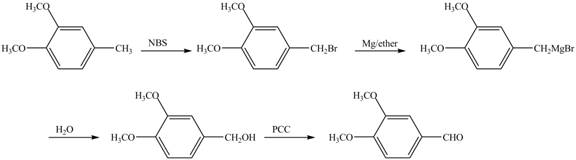
Figure 7
The preparation method of 1,2−dimethoxy−4−benzaldehyde from 1,2−dimethoxy−4−methylbenzene is shown in Figure 7.
(h)
Interpretation:
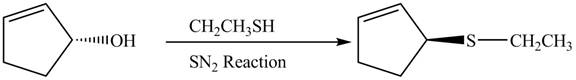
The preparation of given compound from cyclopentenol is to be given.
Concept introduction:
The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as a substitution reaction. In a nucleophilic substitution reaction, nucleophile takes the position of leaving the group by attacking the electron-deficient carbon atom.
Answer to Problem 17.28AP
The preparation of given compound from cyclopentenol is shown below.
Explanation of Solution
The given reaction is a nucleophilic substitution reaction. The nucleophile attacks to replace the OH− group. This is a single step reaction therefore a SN2 reaction.
This is a single step substitution nucleophile reaction as shown below.
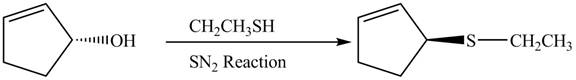
Figure 8
In this reaction, the stereochemistry of molecule changes as from one side the group is leaving and upon other side of the molecule, nucleophile attacks.
The preparation of given compound from cyclopentenol is shown in Figure 8.
Want to see more full solutions like this?
Chapter 17 Solutions
ORGANIC CHEM +SG +SAPLING >IP<
- Identify and provide a concise explanation of a specific analytical instrument capable of detecting and quantifying trace compounds in food samples. Emphasise the instrumental capabilities relevant to trace compound analysis in the nominated food. Include the specific application name (eg: identification and quantification of mercury in salmon), outline a brief description of sample preparation procedures, and provide a summary of the obtained results from the analytical process.arrow_forwardIdentify and provide an explanation of what 'Seperation Science' is. Also describe its importance with the respect to the chemical analysis of food. Provide specific examples.arrow_forward5. Propose a Synthesis for the molecule below. You may use any starting materials containing 6 carbons or less (reagents that aren't incorporated into the final molecule such as PhзP do not count towards this total, and the starting material can have whatever non-carbon functional groups you want), and any of the reactions you have learned so far in organic chemistry I, II, and III. Your final answer should show each step separately, with intermediates and conditions clearly drawn. H3C CH3arrow_forward
- State the name and condensed formula of isooxazole obtained by reacting acetylacetone and hydroxylamine.arrow_forwardState the name and condensed formula of the isothiazole obtained by reacting acetylacetone and thiosemicarbazide.arrow_forwardProvide the semi-developed formula of isooxazole obtained by reacting acetylacetone and hydroxylamine.arrow_forward
- Given a 1,3-dicarbonyl compound (R1-CO-CH2-CO-R2), indicate the formula of the compound obtaineda) if I add hydroxylamine (NH2OH) to give an isooxazole.b) if I add thiosemicarbazide (NH2-CO-NH-NH2) to give an isothiazole.arrow_forwardAn orange laser has a wavelength of 610 nm. What is the energy of this light?arrow_forwardThe molar absorptivity of a protein in water at 280 nm can be estimated within ~5-10% from its content of the amino acids tyrosine and tryptophan and from the number of disulfide linkages (R-S-S-R) between cysteine residues: Ε280 nm (M-1 cm-1) ≈ 5500 nTrp + 1490 nTyr + 125 nS-S where nTrp is the number of tryptophans, nTyr is the number of tyrosines, and nS-S is the number of disulfide linkages. The protein human serum transferrin has 678 amino acids including 8 tryptophans, 26 tyrosines, and 19 disulfide linkages. The molecular mass of the most dominant for is 79550. Predict the molar absorptivity of transferrin. Predict the absorbance of a solution that’s 1.000 g/L transferrin in a 1.000-cm-pathlength cuvet. Estimate the g/L of a transferrin solution with an absorbance of 1.50 at 280 nm.arrow_forward
- In GC, what order will the following molecules elute from the column? CH3OCH3, CH3CH2OH, C3H8, C4H10arrow_forwardBeer’s Law is A = εbc, where A is absorbance, ε is the molar absorptivity (which is specific to the compound and wavelength in the measurement), and c is concentration. The absorbance of a 2.31 × 10-5 M solution of a compound is 0.822 at a wavelength of 266 nm in a 1.00-cm cell. Calculate the molar absorptivity at 266 nm.arrow_forwardHow to calculate % of unknown solution using line of best fit y=0.1227x + 0.0292 (y=2.244)arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





