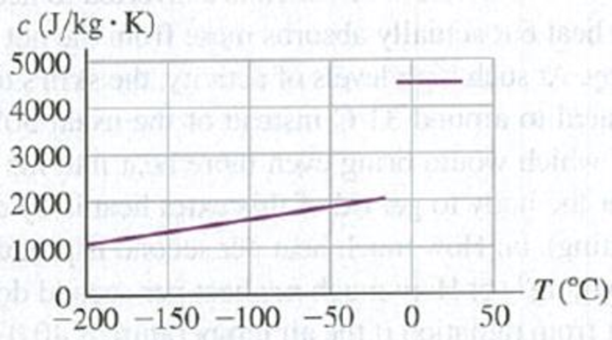
Problem 17.1DQ: Explain why it would not make sense to use a full-size glass thermometer to measure the temperature... Problem 17.2DQ: If you heat the air inside a rigid, scaled container until its Kelvin temperature doubles, the air... Problem 17.3DQ: Many automobile engines have cast-iron cylinders and aluminum pistons. What kinds of problems could... Problem 17.4DQ: Why do frozen water pipes burst? Would a mercury thermometer break if the temperature went below the... Problem 17.5DQ: Two bodies made of the same material have the same external dimensions and appearance, but one is... Problem 17.6DQ: Why is it sometimes possible to loosen caps on screw-top bottles by dipping the capped bottle... Problem 17.7DQ: The inside of an oven is at a temperature of 200C (392F). You can put your hand in the oven without... Problem 17.8DQ: A newspaper article about the weather states that the temperature of a body measures how much heat... Problem 17.9DQ: A student asserts that a suitable unit for specific heat is 1 m2/s2 C. Is she correct? Why or why... Problem 17.10DQ Problem 17.11DQ: The units of specific heat c are J/kg K, but the units of heat of fusion Lf or heat of vaporization... Problem 17.12DQ: Why is a hot, humid day in the tropics generally more uncomfortable for human begins than a hot, dry... Problem 17.13DQ: A piece of aluminum foil used to wrap a potato for baking in a hot oven can usually be handled... Problem 17.14DQ: Desert travelers sometimes keep water in a canvas bag. Some water seeps through the bag and... Problem 17.15DQ: When you first step out of the shower, you feel cold. But as soon as you are dry you feel warmer,... Problem 17.16DQ: The climate of regions adjacent to large bodies of water (like the Pacific and Atlantic coasts)... Problem 17.17DQ: When water is placed in ice-cube trays in a freezer, why doesnt the water freeze all at once when... Problem 17.18DQ: Before giving you an injection, a physician swabs your arm with isopropyl alcohol at room... Problem 17.19DQ: A cold block of metal feels colder than a block of wood at the same temperature. Why? A hot block of... Problem 17.20DQ: A person pours a cup of hot coffee, intending to drink it five minutes later. To keep the coffee as... Problem 17.21DQ: When a freshly baked apple pie has just been removed from the oven, the crust and filling are both... Problem 17.22DQ: Old-time kitchen lore suggests that things cook better (evenly and without burning) in heavy... Problem 17.23DQ: In coastal regions in the winter, the temperature over the land is generally colder than the... Problem 17.24DQ: It is well known that a potato bakes faster if a large nail is stuck through it. Why? Does an... Problem 17.25DQ: Glider pilots in the Midwest know that thermal updrafts are likely to occur in the vicinity of... Problem 17.26DQ: Some folks claim that ice cubes freeze faster if the trays are filled with hot water, because hot... Problem 17.27DQ: Were lucky that the earth isnt in thermal equilibrium with the sun (which has a surface temperature... Problem 17.28DQ Problem 17.1E: Convert the following Celsius temperatures to Fahrenheit: (a) 62.8C, the lowest temperature ever... Problem 17.2E: BIO Temperatures in Biomedicine. (a) Normal body temperature. The average normal body temperature... Problem 17.3E Problem 17.4E: (a) Calculate the one temperature at which Fahrenheit and Celsius thermometers agree with each... Problem 17.5E: You put a bottle of soft drink in a refrigerator and leave it until its temperature has dropped 10.0... Problem 17.6E Problem 17.7E: The pressure of a gas at the triple point of water is 1.35 atm. If its volume remains unchanged,... Problem 17.8E Problem 17.9E Problem 17.10E Problem 17.11E: The Humber Bridge in England has the worlds longest single span, 1410 m. Calculate the change in... Problem 17.12E: One of the tallest buildings in the world is the Taipei 101 in Taiwan, at a height of 1671 feet.... Problem 17.13E: A U.S. penny has a diameter of 1.9000 cm at 20.0C. The coin is made of a metal alloy (mostly zinc)... Problem 17.14E: Ensuring a Tight Fit. Aluminum rivets used in airplane construction are made slightly larger than... Problem 17.15E: A copper cylinder is initially at 20.0C. At what temperature will its volume be 0.150% larger than... Problem 17.16E: A geodesic dome constructed with an aluminum framework is a nearly perfect hemisphere; its diameter... Problem 17.17E: A glass flask whose volume is 1000.00 cm3 at 0.0C is completely filled with mercury at this... Problem 17.18E: A steel tank is completely filled with 1.90 m3 of ethanol when both the tank and the ethanol are at... Problem 17.19E: A machinist bores a hole of diameter 1.35 cm in a steel plate that is at 25.0C. What is the... Problem 17.20E: As a new mechanical engineer for Engines Inc., you have been assigned to design brass pistons to... Problem 17.21E: Steel train rails are laid in 12.0-m-long segments placed end to end. The rails are laid on a winter... Problem 17.22E: A brass rod is 185 cm long and 1.60 cm in diameter. What force must be applied to each end of the... Problem 17.23E: An aluminum tea kettle with mass 1.10 kg and containing 1.80 kg of water is placed on a stove. If no... Problem 17.24E: In an effort to stay awake for an all-night study session, a student makes a cup of coffee by first... Problem 17.25E Problem 17.26E: BIO Heat Loss During Breathing. In very cold weather a significant mechanism for heat loss by the... Problem 17.27E: You are given a sample of metal and asked to determine its specific heat. You weigh the sample and... Problem 17.28E: On-Demand Water Heaters. Conventional hot-water heaters consist of a tank of water maintained at a... Problem 17.29E Problem 17.30E Problem 17.31E: CP A nail driven into a board increases in temperature. If we assume that 60% of the kinetic energy... Problem 17.32E: A technician measures the specific heat of an unidentified liquid by immersing an electrical... Problem 17.33E: CP A 15.0-g bullet traveling horizontally at 865 m/s passes through a tank containing 13.5 kg of... Problem 17.34E: You have 750 g of water at 10.0C in a large insulated beaker. How much boiling water at 100.0C must... Problem 17.35E Problem 17.36E: BIO Treatment for a Stroke. One suggested treatment for a person who has suffered a stroke is... Problem 17.37E: A blacksmith cools a 1.20-kg chunk of iron, initially at 650.0C, by trickling 15.0C water over it.... Problem 17.38E: A copper calorimeter can with mass 0.100 kg contains 0.160 kg of water and 0.0180 kg of ice in... Problem 17.39E: A copper pot with a mass of 0.500 kg contains 0.170 kg of water, and both are at 20.0C. A 0.250-kg... Problem 17.40E: In a container of negligible mass, 0.200 kg of ice at an initial temperature of 40.0C is mixed with... Problem 17.41E Problem 17.42E: BIO Before going in for his annual physical, a 70.0-kg man whose body temperature is 37.0C consumes... Problem 17.43E Problem 17.44E Problem 17.45E: How much heat is required to convert 18.0 g of ice at 10.0C to steam at 100.0C? Express your answer... Problem 17.46E: An open container holds 0.550 kg of ice at 15.0C. The mass of the container can be ignored. Heat is... Problem 17.47E: CP What must the initial speed of a lead bullet be at 25.0C so that the heat developed when it is... Problem 17.48E: BIO Steam Burns Versus Water Burns. What is the amount of heat input to your skin when it receives... Problem 17.49E: BIO The Ship of the Desert. Camels require very little water because they are able to tolerate... Problem 17.50E: BIO Evaporation of sweat is an important mechanism for temperature control in some warm-blooded... Problem 17.51E: CP An asteroid with a diameter of 10 km and a mass of 2.60 l015 kg impacts the earth at a speed of... Problem 17.52E: A laboratory technician drops a 0.0850-kg sample of unknown solid material, at 100.0C, into a... Problem 17.53E: An insulated beaker with negligible mass contains 0.250 kg of water at 75.0C. How many kilograms of... Problem 17.54E: A 4.00-kg silver ingot is taken from a furnace, where its temperature is 750.0C, and placed on a... Problem 17.55E: A vessel whose walls are thermally insulated contains 2.40 kg of water and 0.450 kg of ice, all at... Problem 17.56E Problem 17.57E: Suppose that the rod in Fig. 17.24a is made of copper, is 45.0 cm long, and has a cross-sectional... Problem 17.58E: One end of an insulated metal rod is maintained at 100.0C, and the other end is maintained at 0.00C... Problem 17.59E: A carpenter builds an exterior house wall with a layer of wood 3.0 cm thick on the outside and a... Problem 17.60E: An electric kitchen range has a total wall area of 1.40 m2 and is insulated with a layer of... Problem 17.61E: BIO Conduction Through the Skin. The blood plays an important role in removing heat from the body by... Problem 17.62E: A long rod, insulated to prevent heat loss along its sides, is in perfect thermal contact with... Problem 17.63E: A pot with a steel bottom 8.50 mm thick rests on a hot stove. The area of the bottom of the pot is... Problem 17.64E: You are asked to design a cylindrical steel rod 50.0 cm long, with a circular cross section, that... Problem 17.65E: A picture window has dimensions of 1.40 m 2.50 m and is made of glass 5.20 mm thick. On a winter... Problem 17.66E Problem 17.67E: A spherical pot contains 0.75 L of hot coffee (essentially water) at an initial temperature of 95C.... Problem 17.68E: The emissivity of tungsten is 0.350. A tungsten sphere with radius 1.50 cm is suspended within a... Problem 17.69E: Size of a Light-Bulb Filament. The operating temperature of a tungsten filament in an incandescent... Problem 17.70E: The Sizes of Stars. The hot glowing surfaces of stars emit energy in the form of electromagnetic... Problem 17.71P: CP A Foucault pendulum consists of a brass sphere with a diameter of 35.0 cm suspended from a steel... Problem 17.72P: Suppose that a steel hoop could be constructed to fit just around the earths equator at 20.0C. What... Problem 17.73P: You propose a new temperature scale with temperatures given in M. You define 0.0M to be the normal... Problem 17.74P: CP CALC A 250-kg weight is hanging from the ceiling by a thin copper wire. In its fundamental mode,... Problem 17.75P Problem 17.76P: A surveyors 30.0-m steel tape is correct at 20.0C. The distance between two points, as measured by... Problem 17.77P: A metal rod that is 30.0 cm long expands by 0.0650 cm when its temperature is raised from 0.0C to... Problem 17.78P: On a cool (4.0C) Saturday morning, a pilot fills the fuel tanks of her Pitts S-2C (a two-seat... Problem 17.79P: (a) Equation (17.12) gives the stress required to keep the length of a rod constant as its... Problem 17.80P: CP A metal wire, with density and Youngs modulus Y, is stretched between rigid supports. At... Problem 17.81P: A steel ring with a 2.5000-in. inside diameter at 20.0C is to be warmed and slipped over a brass... Problem 17.82P: BIO Doughnuts: Breakfast of Champions! Atypical doughnut contains 2.0 g of protein, 17.0 g of... Problem 17.83P: BIO Shivering. Shivering is your bodys way of generating heat to restore its internal temperature to... Problem 17.84P: You cool a 100.0-g slug of red-hot iron (temperature 745C) by dropping it into an insulated cup of... Problem 17.85P: CALC Debyes T3 Law. At very low temperatures the molar heat capacity of rock salt varies with... Problem 17.86P: CP A person of mass 70.0 kg is sitting in the bathtub. The bathtub is 190.0 cm by 80.0 cm; before... Problem 17.87P: Hot Air in a Physics Lecture. (a) A typical student listening attentively to a physics lecture has a... Problem 17.88P: CALC The molar heat capacity of a certain substance varies with temperature according to the... Problem 17.89P Problem 17.90P: BIO Overheating. (a) By how much would the body temperature of the bicyclist in Problem 17.89... Problem 17.91P: BIO A Thermodynamic Process in an Insect. The African bombardier beetle (Stenaptinus insignis) can... Problem 17.92P: Hot Water Versus Steam Heating. In a household hot-water heating system, water is delivered to the... Problem 17.93P: You have 1.50 kg of water at 28.0C in an insulated container of negligible mass. You add 0.600 kg of... Problem 17.94P: A thirsty nurse cools a 2.00-L bottle of a soft drink (mostly water) by pouring it into a large... Problem 17.95P Problem 17.96P: A Styrofoam bucket of negligible mass contains 1.75 kg of water and 0.450 kg of ice. More ice, from... Problem 17.97P: In a container of negligible mass, 0.0400 kg of steam at 100C and atmospheric pressure is added to... Problem 17.98P Problem 17.99P: Effect of a Window in a Door. A carpenter builds a solid wood door with dimensions 2.00 m 0.95 m ... Problem 17.100P: One experimental method of measuring an insulating materials thermal conductivity is to construct a... Problem 17.101P: Compute the ratio of the rate of heat loss through a single-pane window with area 0.15 m2 to that... Problem 17.102P: Rods of copper, brass, and steeleach with cross-sectional area of 2.00 cm2are welded together to... Problem 17.103P: A brass rod 12.0 cm long, a copper rod 18.0 cm long, and an aluminum rod 24.0 cm longeach with... Problem 17.104P: BIO Basal Metabolic Rate. The basal metabolic rate is the rate at which energy is produced in the... Problem 17.105P Problem 17.106P Problem 17.107P: A Thermos for Liquid Helium. A physicist uses a cylindrical metal can 0.250 m high and 0.090 m in... Problem 17.108P: A metal sphere with radius 3.20 cm is suspended in a large metal box with interior walls that are... Problem 17.109P Problem 17.110P: The icecaps of Greenland and Antarctica contain about 1.75% of the total water (by mass) on the... Problem 17.111P: DATA As a physicist, yon put heat into a 500.0-g solid sample at the rate of 10.0 kJ/min while... Problem 17.112P: DATA At a chemical plant where you are an engineer, a tank contains an unknown liquid. You must... Problem 17.113P: DATA During your mechanical engineering internship, you are given two uniform metal bars A and B,... Problem 17.114CP Problem 17.115CP: A hollow cylinder has length L, inner radius a, and outer radius b, and the temperatures at the... Problem 17.116PP: You place 35 g of this cryoprotectant at 22C in contact with a cold plate that is maintained at the... Problem 17.117PP: Careful measurements show that the specific heat of the solid phase depends on temperature (Fig.... Problem 17.118PP: In another experiment, you place a layer of this cryoprotectant between one 10 cm 10 cm cold plate... Problem 17.119PP: To measure the specific heat in the liquid phase of a newly developed cryoprotectant, you place a... format_list_bulleted


 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
 Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning




