
Laboratory Experiments for Chemistry: The Central Science (13th Edition)
13th Edition
ISBN: 9780321949912
Author: Theodore E. Brown, John H. Nelson, Kenneth C. Kemp
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 17, Problem 11E
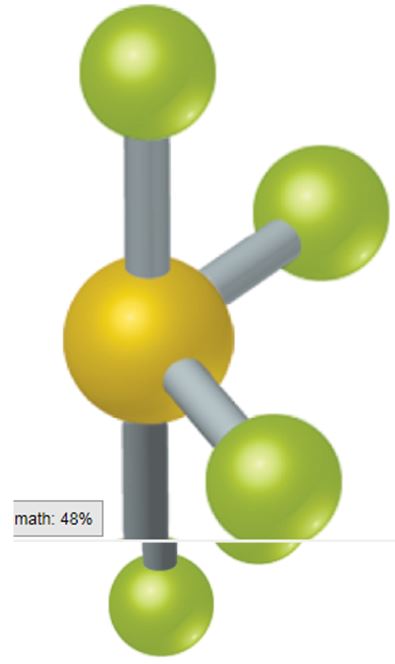
9.1 A certain AB4, molecule has a "seesaw" shape
From which of the fundamental geometries shown in Figure 9.3could you remove one or more
atoms to create a molecule having this seesaw shape? (Section 9.1
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
There is an instrument in Johnson 334 that measures total-reflectance x-ray fluorescence (TXRF) to do elemental analysis (i.e., determine what elements are present in a sample). A researcher is preparing a to measure calcium content in a series of well water samples by TXRF with an internal standard of vanadium (atomic symbol: V). She has prepared a series of standard solutions to ensure a linear instrument response over the expected Ca concentration range of 40-80 ppm. The concentrations of Ca and V (ppm) and the instrument response (peak area, arbitrary units) are shown below. Also included is a sample spectrum. Equation 1 describes the response factor, K, relating the analyte signal (SA) and the standard signal (SIS) to their respective concentrations (CA and CIS).
Ca, ppm
V, ppm
SCa, arb. units
SV, arb. units
20.0
10.0
14375.11
14261.02
40.0
10.0
36182.15
17997.10
60.0
10.0
39275.74
12988.01
80.0
10.0
57530.75
14268.54
100.0…
A mixture of 0.568 M H₂O, 0.438 M Cl₂O, and 0.710 M HClO are enclosed in a vessel at 25 °C.
H₂O(g) + C₁₂O(g) = 2 HOCl(g)
K = 0.0900 at 25°C
с
Calculate the equilibrium concentrations of each gas at 25 °C.
[H₂O]=
[C₁₂O]=
[HOCI]=
M
Σ
M
What units (if any) does the response factor (K) have? Does the response factor (K) depend upon how the concentration is expressed (e.g. molarity, ppm, ppb, etc.)?
Chapter 17 Solutions
Laboratory Experiments for Chemistry: The Central Science (13th Edition)
Ch. 17.1 - Calculate the formal charge on the indicated atom...Ch. 17.1 - The hypochlorite ion, CIO- , is the active...Ch. 17.1 - Prob. 17.2.1PECh. 17.1 - a. Triazine, C3 H3N3, is like benzene except that...Ch. 17.2 - Prob. 17.3.1PECh. 17.2 - Prob. 17.3.2PECh. 17.2 - Prob. 17.4.1PECh. 17.2 - Prob. 17.4.2PECh. 17.2 - Prob. 17.5.1PECh. 17.2 - Prob. 17.5.2PE
Ch. 17.2 - Prob. 17.6.1PECh. 17.2 - Prob. 17.6.2PECh. 17.3 - Prob. 17.7.1PECh. 17.3 -
8.103 The compound chloral hydrate, known in...Ch. 17.3 - Barium azide is 62.04% Ba and 37.96% N. Each azide...Ch. 17.3 - Acetylene (C2H2) and nitrogen (N2) both contain a...Ch. 17.3 - Prob. 17.9.1PECh. 17.3 - Prob. 17.9.2PECh. 17.4 - Prob. 17.10.1PECh. 17.4 - Prob. 17.10.2PECh. 17.4 - Prob. 17.11.1PECh. 17.4 - A new compound is made that has a C-C bond length...Ch. 17.4 - A new compound is made that has an N-N bond length...Ch. 17.4 - Prob. 17.12.2PECh. 17.5 - Prob. 17.13.1PECh. 17.5 - An ionic substance of formula MX has a lattice...Ch. 17.5 - Prob. 17.14.1PECh. 17.5 - Prob. 17.14.2PECh. 17.5 - Prob. 17.15.1PECh. 17.5 - Consider the collection of nonmetallic elements 0,...Ch. 17.6 - The substance chlorine monoxide, CIO(g), is...Ch. 17.6 -
[8.87]
a. using the electronegativities of Br...Ch. 17.6 - Prob. 17.17.1PECh. 17.6 - Although I3- is a known ion, F3- is not. a. Draw...Ch. 17 - Prob. 1DECh. 17 -
9.13
a. An AB2 molecule is linear. How...Ch. 17 - Give the electron-domain and molecular geometries...Ch. 17 - Prob. 3ECh. 17 - Prob. 4ECh. 17 - Prob. 5ECh. 17 - Prob. 6ECh. 17 - Prob. 7ECh. 17 - Prob. 8ECh. 17 - Azo dyes are organic dyes that are used for many...Ch. 17 - Prob. 10ECh. 17 - 9.1 A certain AB4, molecule has a "seesaw" shape...Ch. 17 - Prob. 12ECh. 17 - Prob. 13ECh. 17 - Prob. 14ECh. 17 - Prob. 15ECh. 17 - In the hydrocarbon a. What is the hybridization at...Ch. 17 - The drawing below shows the overlap of two hybrid...Ch. 17 - Prob. 18ECh. 17 -
9.10 The following is part of a molecular...Ch. 17 - a. Methane (CH4) and the perchlorate ion (C104-)...Ch. 17 - Prob. 21ECh. 17 - Prob. 22ECh. 17 - Prob. 23ECh. 17 - Prob. 24ECh. 17 - In which of these molecules or ions does the...Ch. 17 - Prob. 26ECh. 17 - How many nonbonding electron pairs are there in...Ch. 17 - Prob. 28ECh. 17 - Prob. 29ECh. 17 - Prob. 30ECh. 17 - Prob. 31ECh. 17 - Prob. 32ECh. 17 - Prob. 33ECh. 17 - Prob. 34ECh. 17 - Ammonia, NH3 reacts with incredibly strong bases...Ch. 17 - In which of the following AFn molecules or ions is...Ch. 17 - a. Explain why BrF4 is square planar, whereas...Ch. 17 -
9.34 Name the proper three-dimensional molecule...Ch. 17 - Prob. 39ECh. 17 - Prob. 40ECh. 17 - a. (a) Is the molecule BF3 polar or nonpolar? b....Ch. 17 - Prob. 42ECh. 17 - Predict whether each of the following molecules is...Ch. 17 - Prob. 44ECh. 17 - Prob. 45ECh. 17 - Prob. 46ECh. 17 - For each statement, irldicate whether it is true...Ch. 17 - Draw sketches illustrating the overlap between the...Ch. 17 - For each statement, indicate whether it is true or...Ch. 17 - Consider the SC12 molecule. a. What IS the...Ch. 17 - Prob. 51ECh. 17 - Prob. 52ECh. 17 - Prob. 53ECh. 17 - Prob. 54ECh. 17 - Prob. 55ECh. 17 - Prob. 56ECh. 17 - a. Draw Lewis structures for ethane (C2He),...Ch. 17 - a. Draw Lewis structures for ethane (C2He),...Ch. 17 - Prob. 59ECh. 17 - Prob. 60ECh. 17 - Prob. 61ECh. 17 - Prob. 62ECh. 17 - In the formate ion, HC02- , the carbon atom is the...Ch. 17 - Prob. 64ECh. 17 - Prob. 65ECh. 17 - Prob. 66ECh. 17 - Prob. 67ECh. 17 - a. If you combine two atomic orbitals on two...Ch. 17 - Prob. 69ECh. 17 - Indicate whether each statement is true or false....Ch. 17 - Prob. 71ECh. 17 - Prob. 72ECh. 17 - Prob. 73ECh. 17 - Prob. 74ECh. 17 - Prob. 75ECh. 17 - Prob. 76ECh. 17 - Determine the electron configurations for CN+, CN,...Ch. 17 - Prob. 78ECh. 17 - Consider the molecular orbitals of the P2...Ch. 17 - Prob. 80ECh. 17 - Consider the following XF4 ions: PF4, BrF4-,...Ch. 17 -
9.88 Consider the molecule PF4Cl....Ch. 17 - Prob. 83AECh. 17 - Fill in the blank spaces in the following chart....Ch. 17 - Prob. 85AECh. 17 - Prob. 86AECh. 17 - Prob. 87AECh. 17 - Prob. 88AECh. 17 - Prob. 89AECh. 17 - Prob. 90AECh. 17 - Prob. 91AECh. 17 - Prob. 92AECh. 17 - In ozone, 03, the two oxygen atoms on the ends Of...Ch. 17 - Butadiene, C4H6, is a planar molecule that has the...Ch. 17 - The structure of borazine, B3N3H6, is a...Ch. 17 - Prob. 96AECh. 17 - Prob. 97AECh. 17 - Prob. 98AECh. 17 - Prob. 99AECh. 17 - Prob. 100AECh. 17 - Prob. 101AECh. 17 - Consider the following AB3 molecules and ions-...Ch. 17 - Prob. 103AECh. 17 - Prob. 104AECh. 17 - Prob. 105AECh. 17 - Prob. 106AECh. 17 - Prob. 107AECh. 17 - Prob. 108AECh. 17 - Determine whether the following molecules are...Ch. 17 - Prob. 110IECh. 17 - Prob. 111IECh. 17 - Prob. 112IECh. 17 - Prob. 113IECh. 17 - Prob. 114IECh. 17 - Prob. 115IECh. 17 - Prob. 116IECh. 17 - Prob. 117IECh. 17 - Prob. 118IECh. 17 - Prob. 119IECh. 17 - Prob. 120IE
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Provide the structure, circle or draw, of the monomeric unit found in the biological polymeric materials given below. HO OH amylose OH OH 행 3 HO cellulose OH OH OH Ho HOarrow_forwardWhat units (if any) does K have? Does K depend upon how the concentration is expressed (e.g. molarity, ppm, ppb, etc.)? in calculating the response factorarrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forward
- Don't used Ai solution and don't used hand raitingarrow_forwardOA. For the structure shown, rank the bond lengths (labeled a, b and c) from shortest to longest. Place your answer in the box. Only the answer in the box will be graded. (2 points) H -CH3 THe b Нarrow_forwardDon't used hand raitingarrow_forward
- Quizzes - Gen Organic & Biological Che... ☆ myd21.lcc.edu + O G screenshot on mac - Google Search savings hulu youtube google disney+ HBO zlib Homework Hel...s | bartleby cell bio book Yuzu Reader: Chemistry G periodic table - Google Search b Home | bartleby 0:33:26 remaining CHEM 120 Chapter 5_Quiz 3 Page 1: 1 > 2 > 3 > 6 ¦ 5 > 4 > 7 ¦ 1 1 10 8 ¦ 9 a ¦ -- Quiz Information silicon-27 A doctor gives a patient 0.01 mC i of beta radiation. How many beta particles would the patient receive in I minute? (1 Ci = 3.7 x 10 10 d/s) Question 5 (1 point) Saved Listen 2.22 x 107 222 x 108 3.7 x 108 2.22 x 108 none of the above Question 6 (1 point) Listen The recommended dosage of 1-131 for a test is 4.2 μCi per kg of body mass. How many millicuries should be given to a 55 kg patient? (1 mCi = 1000 μСi)? 230 mCiarrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forward
- Q3: Arrange each group of compounds from fastest SN2 reaction rate to slowest SN2 reaction rate. CI Cl H3C-Cl CI a) A B C D Br Br b) A B C Br H3C-Br Darrow_forwardQ4: Rank the relative nucleophilicity of halide ions in water solution and DMF solution, respectively. F CI Br | Q5: Determine which of the substrates will and will not react with NaSCH3 in an SN2 reaction to have a reasonable yield of product. NH2 Br Br Br .OH Brarrow_forwardClassify each molecule as optically active or inactive. Determine the configuration at each H соон Chirality center OH 애 He OH H3C Ноос H H COOH A K B.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning
Linear Combination of Atomic Orbitals LCAO; Author: Edmerls;https://www.youtube.com/watch?v=nq1zwrAIr4c;License: Standard YouTube License, CC-BY
Quantum Molecular Orbital Theory (PChem Lecture: LCAO and gerade ungerade orbitals); Author: Prof Melko;https://www.youtube.com/watch?v=l59CGEstSGU;License: Standard YouTube License, CC-BY