
a) Bromobenzene
Interpretation:
The major product(s) formed when bromobenzene is nitrated is to be given. Whether it will react faster or slower than benzene is also to be stated.
Concept introduction:
Monosubstituted benzenes can be nitrated using a mixture of Conc. HNO3 and H2SO4. Electron releasing substituent groups, excep halogens, activate the ring and direct the incoming electrophile to the o- and p-positions. Compounds with these substituent groups are more reactive than benzene. Halogens are o- and p-directors but they deactivate the ring. Hence halobenzenes are less reactive than benzene. Electron withdrawing substituent groups deactivate the ring and direct the incoming electrophile to the m-position. Compounds with these substituent groups are less reactive than benzene.
To give:
The major products formed when bromobenzene is nitrated and to state whether it will react faster or slower than benzene.
Answer to Problem 47AP
The major products formed when bromobenzene is nitrated are o-bromonitrobenzene and p-bromonitrobenzene.
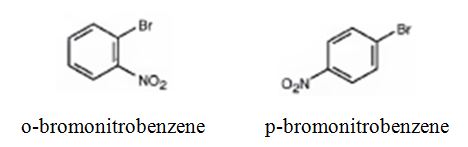
Bromobenzene will react slower than benzene.
Explanation of Solution
Bromine is an o- and p-directing group. It also has considerable electron withdrawing inductive effect which deactivates the ring. Hence bromobenzene is less reactive than benzene.
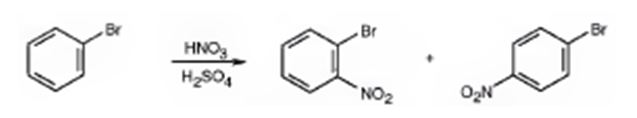
The major products formed when bromobenzene is nitrated are o-bromonitrobenzene and p-bromonitrobenzene.
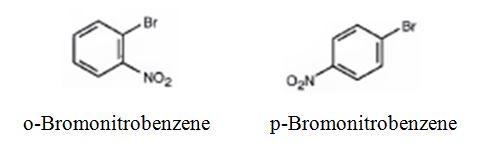
Bromobenzene will react slower than benzene.
b) Benzonitrile
Interpretation:
The major product(s) formed when benzonitrile is nitrated is to be given. Whether it will react faster or slower than benzene is also to be stated.
Concept introduction:
Monosubstituted benzenes can be nitrated using a mixture of Conc. HNO3 and H2SO4. Electron releasing substituent groups, except halogens, activate the ring and direct the incoming electrophile to the o- and p-positions. Compounds with these substituents are more reactive than benzene. Halogens are o- and p-directors but they deactivate the ring. Hence halobenzenes are less reactive than benzene. Electron withdrawing substituent groups deactivate the ring and direct the incoming electrophile to the m-position. Compounds with these substituents are less reactive than benzene.
To give:
The major products formed when benzonitrile is nitrated and to state whether it will react faster or slower than benzene.
Answer to Problem 47AP
The major product formed when benzonitrile is nitrated is m-nitrobenzonitrile.
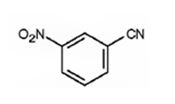
Benzonitrile will react slower than benzene.
Explanation of Solution
The cyanide group is strongly electron withdrawing in nature. Hence it is a meta director. The attraction of electrons away from the ring reduces the electron density in the ring. Thus benzonitrile reacts slower than benzene.
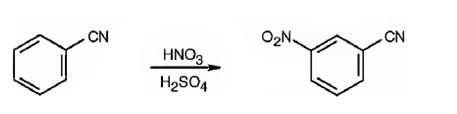
The major product formed when benzonitrile is nitrated is m-nitrobenzonitrile.

Benzonitrile will react slower than benzene.
c) Benzoic acid
Interpretation:
The major product(s) formed when benzoic acid is nitrated is to be given. Whether it will react faster or slower than benzene is also to be stated.
Concept introduction:
Monosubstituted benzenes can be nitrated using a mixture of Conc. HNO3 and H2SO4. Electron releasing substituent groups, except halogens, activate the ring and direct the incoming electrophile to the o- and p-positions. Compounds with these substituents are more reactive than benzene. Halogens are o- and p-directors but they deactivate the ring. Hence halobenzenes are less reactive than benzene. Electron withdrawing substituent groups deactivate the ring and direct the incoming electrophile to the m-position. Compounds with these substituents are less reactive than benzene.
To give:
The major products formed when benzoic acid is nitrated and to state whether it will react faster or slower than benzene.
Answer to Problem 47AP
The major product formed when benzoic acid is nitrated is m-nitrobenzoic acid.
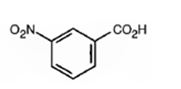
Benzoic acid will react slower than benzene.
Explanation of Solution
The C=O in carboxyl group is strongly electron withdrawing in nature. Hence it is a meta director. The attraction of electrons away from the ring reduces the electron density in the ring. Thus benzoic acid reacts slower than benzene.
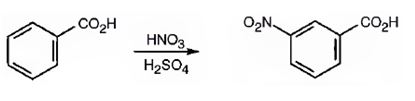
The major product formed when benzoic acid is nitrated is m-nitrobenzoic acid.

Benzoic acid will react slower than benzene.
d) Nitrobenzene
Interpretation:
The major product(s) formed when nitrobenzene is nitrated is to be given. Whether it will react faster or slower than benzene is also to be stated.
Concept introduction:
Monosubstituted benzenes can be nitrated using a mixture of Conc. HNO3 and H2SO4. Electron releasing substituent groups, except halogens, activate the ring and direct the incoming electrophile to the o- and p-positions. Compounds with these substituents are more reactive than benzene. Halogens are o- and p-directors but they deactivate the ring. Hence halobenzenes are less reactive than benzene. Electron withdrawing substituent groups deactivate the ring and direct the incoming electrophile to the m-position. Compounds with these substituents are less reactive than benzene.
To give:
The major products formed when nitrobenzene is nitrated and to state whether it will react faster or slower than benzene.
Answer to Problem 47AP
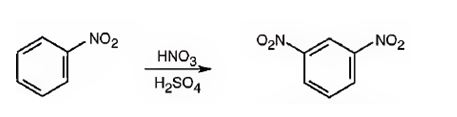
The major product formed when nitrobenzene is nitrated is m-dinitrobenzene.
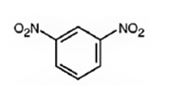
Nitrobenzene will react slower than benzene.
Explanation of Solution
The nitro group is strongly electron withdrawing in nature. Hence it is a meta director. The attraction of electrons away from the ring reduces the electron density in the ring. Thus nitrobenzene reacts slower than benzene.
The major product formed when nitrobenzene is nitrated is m-dinitrobenzene.

Nitrobenzene will react slower than benzene.
e) Benzenesulfonic acid
Interpretation:
The major product(s) formed when benzenesulfonic acid is nitrated is to be given. Whether it will react faster or slower than benzene is also to be stated.
Concept introduction:
Monosubstituted benzenes can be nitrated using a mixture of Conc. HNO3 and H2SO4. Electron releasing substituent groups, except halogens, activate the ring and direct the incoming electrophile to the o- and p-positions. Compounds with these substituents are more reactive than benzene. Halogens are o- and p-directors but they deactivate the ring. Hence halobenzenes are less reactive than benzene. Electron withdrawing substituent groups deactivate the ring and direct the incoming electrophile to the m-position. Compounds with these substituents are less reactive than benzene.
To give:
The major products formed when benzenesulfonic acid is nitrated and to state whether it will react faster or slower than benzene.
Answer to Problem 47AP
The major product formed when benzenesulfonic acid is nitrated is m-nitro benzenesulfonic acid.
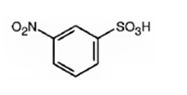
Benzenesulfonic acid will react slower than benzene.
Explanation of Solution
The sulfonic acid group is strongly electron withdrawing in nature. Hence it is a meta director. The attraction of electrons away from the ring reduces the electron density in the ring. Thus benzenesulfonic acid reacts slower than benzene.
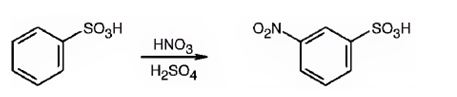
The major product formed when benzenesulfonic acid is nitrated is m-nitro benzenesulfonic acid.

Benzenesulfonic acid will react slower than benzene.
f) Methoxybenzene
Interpretation:
The major product(s) formed when methoxybenzene is nitrated is to be given. Whether it will react faster or slower than benzene is also to be stated.
Concept introduction:
Monosubstituted benzenes can be nitrated using a mixture of Conc. HNO3 and H2SO4. Electron releasing substituent groups, except halogens, activate the ring and direct the incoming electrophile to the o- and p-positions. Compounds with these substituents are more reactive than benzene. Halogens are o- and p-directors but they deactivate the ring. Hence halobenzenes are less reactive than benzene. Electron withdrawing substituent groups deactivate the ring and direct the incoming electrophile to the m-position. Compounds with these substituents are less reactive than benzene.
To give:
The major products formed when methoxybenzene is nitrated and to state whether it will react faster or slower than benzene.
Answer to Problem 47AP
The major products formed when methoxybenzene is nitrated are o-nitromethoxybenene and p-nitromethoxybenene.
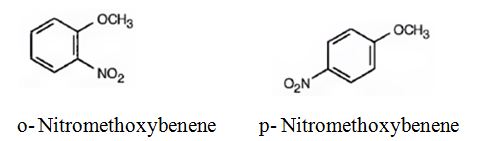
Methoxybenzene will react faster than benzene.
Explanation of Solution
The methoxy group is electron releasing in nature. Hence it is an o- and p-director. The attraction of electrons towards the ring increases the electron density in the ring. Thus methoxybenzene reacts faster than benzene.
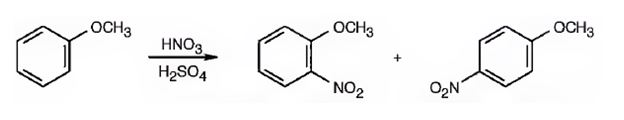
The major products formed when methoxybenzene is nitrated are o-nitromethoxybenene and p-nitromethoxybenene.
Methoxybenzene will react faster than benzene.
Want to see more full solutions like this?
Chapter 16 Solutions
ORGANIC CHEMISTRY-EBOOK>I<
- All of the following are allowed energy levels except _. a) 3f b) 1s c) 3d d) 5p e) 6sarrow_forwardA student wants to make the following product in good yield from a single transformation step, starting from benzene. Add any organic reagents the student is missing on the left-hand side of the arrow, and any addition reagents that are necessary above or below the arrow. If this product can't be made in good yield with a single transformation step, check the box below the drawing area. Note for advanced students: you may assume that an excess of benzene is used as part of the reaction conditions. : ☐ + I X This product can't be made in a single transformation step.arrow_forwardPredict the major products of this organic reaction:arrow_forward
- Name the family to which each organic compound belongs. The first answer has been filled in for you. compound CH₂ || CH3-C-NH2 0 ။ CH3-C-CH₂ CH=O–CH=CH, CH₂ HO CH2-CH2-CH-CH3 family amine Darrow_forward1b. Br LOHarrow_forwardI would like my graphs checked please. Do they look right? Do I have iodine and persulfate on the right axis ?arrow_forward
- Reaction Fill-ins Part 2! Predict the product(s) OR starting material of the following reactions. Remember, Hydride shifts are possible if/when a more stable carbocation can exist (depending on reaction mechanism)! Put your answers in the indicated boxes d. d. ง HCIarrow_forwardA cylinder contains 12 L of water vapour at 150˚C and 5 atm. The temperature of the water vapour is raised to 175˚C, and the volume of the cylinder is reduced to 8.5 L. What is the final pressure of the gas in atmospheres? assume that the gas is idealarrow_forwardOn the next page is an LC separation of the parabens found in baby wash. Parabens are suspected in a link to breast cancer therefore an accurate way to quantitate them is desired. a. In the chromatogram, estimate k' for ethyl paraben. Clearly indicate what values you used for all the terms in your calculation. b. Is this a "good" value for a capacity factor? Explain. c. What is the resolution between n-Propyl paraben and n-Butyl paraben? Again, indicate clearly what values you used in your calculation. MAU | Methyl paraben 40 20 0 -2 Ethyl paraben n-Propyl paraben n-Butyl paraben App ID 22925 6 8 minarrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

