
a)
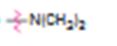
Interpretation:
Whether N,N-dimethylamino group is an activator or deactivator and whether it is a o-, p-director or m-director is to be stated.
Concept introduction:
In
Electron releasing groups, except halogens, normally are activators and ortho & para directors. Electron withdrawing groups normally are deactivators and meta directors. Halogens are ortho & para directing but are deactivating.
To state:
Whether N,N-dimethylamino group is an activator or deactivator and whether it is a, o-, p-director or m-director.
b)
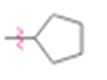
Interpretation:
Whether cyclopentyl group is an activator or deactivator and whether it is an o-, p-director or m-director is to be stated.
Concept introduction:
In aromatic substitution reactions the activating or deactivating and orienting effects of a substituent attached to the benzene ring can be decided from its resonance and inductive effects. The orientation is decided by the resonance effect while the activating or deactivating effect is decided both by resonance and inductive effects. Both effects may reinforce or oppose each other. However the resonance effect is much stronger than the inductive effect.
Electron releasing groups, except halogens, normally are activators and ortho & para directors. Electron withdrawing groups normally are deactivators and meta directors. Halogens are ortho & para directing but are deactivating.
To state:
Whether cyclopentyl group is an activator or deactivator and whether it is an o-, p-director or m-director.
c)
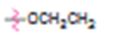
Interpretation:
Whether ethoxy group is an activator or deactivator and whether it is an o-, p-director or m-director is to be stated.
Concept introduction:
In aromatic substitution reactions the activating or deactivating and orienting effects of a substituent attached to the benzene ring can be decided from its resonance and inductive effects. The orientation is decided by the resonance effect while the activating or deactivating effect is decided both by resonance and inductive effects. Both effects may reinforce or oppose each other. However the resonance effect is much stronger than the inductive effect.
Electron releasing groups, except halogens, normally are activators and ortho & para directors. Electron withdrawing groups normally are deactivators and meta directors. Halogens are ortho & para directing but are deactivating.
To state:
Whether ethoxy group is an activator or deactivator and whether it is an o-, p-director or m-director.
d)
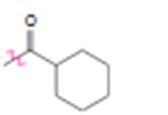
Interpretation:
Whether the carbonyl group is an activator or deactivator and whether it is a o-, p-director or m-director is to be stated.
Concept introduction:
In aromatic substitution reactions the activating or deactivating and orienting effects of a substituent attached to the benzene ring can be decided from its resonance and inductive effects. The orientation is decided by the resonance effect while the activating or deactivating effect is decided both by resonance and inductive effects. Both effects may reinforce or oppose each other. However the resonance effect is much stronger than the inductive effect.
Electron releasing groups, except halogens, normally are activators and ortho & para directors. Electron withdrawing groups normally are deactivators and meta directors. Halogens are ortho & para directing but are deactivating.
To state:
Whether carbonyl group is an activator or deactivator and whether it is a o-, p-director or m-director.
Trending nowThis is a popular solution!

Chapter 16 Solutions
EBK ORGANIC CHEMISTRY
- When anisole is treated with excess bromine, the reaction gives a product which shows two singlets in 1H NMR. Draw the product.arrow_forward(ii) Draw a reasonable mechanism for the following reaction: CI NaOH heat OH (hint: SNAr Reaction) :arrow_forwardDraw the major product in each of the following reaction:arrow_forward
- Draw the mechanism for the following Friedel-Craft reaction. AlBr3 Brarrow_forward(a) Draw the structures of A and B in the following reaction. (i) NaNH2, NH3(1) A + B (ii) H3O+arrow_forwardFor the reaction 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 →> NO₂+ NO3_(K1) NO2 + NO3 → N2O5 (k-1) NO2 + NO3 → → NO2 + O2 + NO (K2) NO + N2O5- NO2 + NO2 + NO2 (K3) d[N₂O5] __2k‚k₂[N2O5] Indicate whether the following rate expression is acceptable: dt k₁₁+ k₂arrow_forward
- Consider the following decomposition reaction of N2O5(g): For the reaction 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 → NO2 + NO3 (K1) NO2 + NO3 → N2O5 (k-1) NO2 + NO3 → NO2 + O2 + NO (K2) NO + N2O5 → NO2 + NO2 + NO2 (K3) Indicate whether the following rate expression is acceptable: d[N2O5] = -k₁[N₂O₂] + K¸₁[NO₂][NO3] - K¸[NO₂]³ dtarrow_forwardIn a reaction of A + B to give C, another compound other than A, B or C may appear in the kinetic equation.arrow_forwardFor the reaction 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 →> NO₂+ NO3_(K1) NO2 + NO3 → N2O5 (k-1) NO2 + NO3 → → NO2 + O2 + NO (K2) NO + N2O5- NO2 + NO2 + NO2 (K3) d[N₂O5] __2k‚k₂[N2O5] Indicate whether the following rate expression is acceptable: dt k₁₁+ k₂arrow_forward
- Given the reaction R + Q → P, indicate the rate law with respect to R, with respect to P and with respect to P.arrow_forwardSteps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forwardk₁ Given the reaction A B, indicate k-1 d[A] (A). the rate law with respect to A: (B). the rate law with respect to B: d[B] dt dtarrow_forward

 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


