
BURDGE CHEMISTRY VALUE ED (LL)
4th Edition
ISBN: 9781259995958
Author: VALUE EDITION
Publisher: MCG CUSTOM
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 16, Problem 7QP
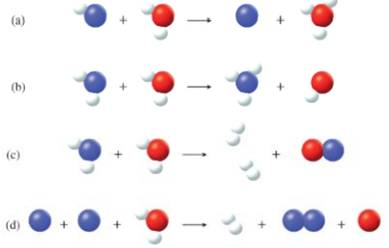
Which of the following could represent a Brønsted acid-base reaction?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
First image: Why can't the molecule C be formed in those conditions
Second image: Synthesis for lactone C
its not an exam
First image: I have to show the mecanism for the reaction on the left, where the alcohol A is added fast in one portion
Second image: I have to show the mecanism of the reaction at the bottom. Also I have to show by mecanism why the reaction wouldn't work if the alcohol was primary
First image: I have to explain why the molecule C is never formed in those conditions.
Second image: I have to propose a synthesis for the lactone A
Chapter 16 Solutions
BURDGE CHEMISTRY VALUE ED (LL)
Ch. 16.1 - Practice Problem ATTEMPT What is (a) the conjugate...Ch. 16.1 - Practice Problem BUILD HSO 3 − is the conjugate...Ch. 16.1 - Practice Problem CONCEPTUALIZE Which of the models...Ch. 16.1 - 16.1.1 Which of the following pairs of species are...Ch. 16.1 - Which of the following species does not have a...Ch. 16.2 - Practice Problem ATTEMPT Identify and label the...Ch. 16.2 - Practice Problem BUILD
(a) Write an equation in...Ch. 16.2 - Practice Problem CONCEPTUALIZE Write the formula...Ch. 16.2 - Calculate [ OH − ] in a solution in which [ H 3 O...Ch. 16.2 - Calculate [ H 3 O − ] in a solution in which [ OH...
Ch. 16.3 - Prob. 1PPACh. 16.3 - Practice Problem BUILD
The value of at normal...Ch. 16.3 - Prob. 1PPCCh. 16.3 - Determine the pH of a solution at 25°C in which [...Ch. 16.3 - 16.3.2 Determine in a solution at...Ch. 16.3 - Determine the pOH of a solution at 25°C in which [...Ch. 16.3 - Determine [ OH − ] in a solution at 25°C if pH =...Ch. 16.4 - Practice ProblemATTEMPT Determine the pH of a...Ch. 16.4 - Practice Problem BUILD
Determine the pH of a...Ch. 16.4 - Practice Problem CONCEPTUALIZE
Strong acid is...Ch. 16.4 - Calculate the pH of a 0.075–-M solution of...Ch. 16.4 - 16.4.2 What is the concentration of in a solution...Ch. 16.4 - 16.4.3 What is the of a solution at that is...Ch. 16.4 - What is the concentration of KOH in a solution at...Ch. 16.4 - What is the pH of a solution at 25°C that is...Ch. 16.4 - What is the concentration of Ca ( OH ) 2 in a...Ch. 16.4 - Which diagram best represents a solution of...Ch. 16.5 - Practice Problem ATTEMPT Calculate the hydronium...Ch. 16.5 - Practice Problem BUILD Calculate the hydroxide ion...Ch. 16.5 - Practice Problem CONCEPTUALIZE
What is the value...Ch. 16.5 - The K a of a weak acid is 5.5 × 10 − 4 . What is...Ch. 16.5 - A 0.042-M solution of a weak acid has pH 4.01 at...Ch. 16.5 - The diagrams show solutions of three different...Ch. 16.6 - Practice ProblemATTEMPT Determine the pOH of a...Ch. 16.6 - Practice Problem BUILD Determine the pH of a...Ch. 16.6 - Prob. 1PPCCh. 16.6 - What is the pH of a 0.63-M solution of weak base...Ch. 16.6 - A 0.12-M solution of a weak base has a pH of 10.76...Ch. 16.6 - The diagrams show solutions of three different...Ch. 16.7 - Practice Problem ATTEMPT
Calculate the hydroxide...Ch. 16.7 - Practice ProblemBUILD Calculate the hydronium ion...Ch. 16.7 - Practice ProblemCONCEPTUALIZE What is the value of...Ch. 16.7 - 16.7.1 Calculate the of the cyanide ion . (See...Ch. 16.7 - Which of the anions listed is the strongest base?...Ch. 16.7 - The diagrams show solutions of three different...Ch. 16.8 - Practice Problem ATTEMPT
Calculate the pH of an...Ch. 16.8 - Practice ProblemBUILD Calculate the pOH of an...Ch. 16.8 - Practice Problem CONCEPTUALIZE
Estimate the pH of...Ch. 16.8 - Calculate the equilibrium concentration of CO 3 2...Ch. 16.8 - What is the pH of a 0.40-M solution of phosphoric...Ch. 16.8 - List the molecular and ionic species in order of...Ch. 16.8 - Which is true for any polyprotic acid? a) K a2 > K...Ch. 16.9 - Practice Problem ATTEMPT Calculate the...Ch. 16.9 - Practice Problem BUILD Calculate the concentration...Ch. 16.9 - Practice Problem CONCEPTUALIZE
Which of the plots...Ch. 16.10 - Practice Problem ATTEMPT Calculate the pOH of the...Ch. 16.10 - Practice Problem BUILD Calculate the pH of the...Ch. 16.10 - Practice ProblemCONCEPTUALIZE Which of the...Ch. 16.10 - Calculate the pH of a 0.075-M solution of...Ch. 16.10 - Calculate the pH of a 0.082-M solution of...Ch. 16.10 - Prob. 3CPCh. 16.10 - Prob. 4CPCh. 16.10 - The diagrams represent solutions of three salts...Ch. 16.11 - Practice Problem ATTEMPT An aqueous solution of a...Ch. 16.11 - Practice Problem BUILD An aqueous solution of a...Ch. 16.11 - Practice ProblemCONCEPTUALIZE Which of the...Ch. 16.12 - Practice Problem ATTEMPT
Calculate the pH at of a...Ch. 16.12 - Practice ProblemBUILD Calculate the pH at 25°C of...Ch. 16.12 - Practice Problem CONCEPTUALIZE The diagrams show...Ch. 16.12 - 16.12.1 Which of the following cannot act as a...Ch. 16.12 - Which of the following is a Lewis acid but not a...Ch. 16.13 - Practice Problem ATTEMPT
Determine the pH and...Ch. 16.13 - Prob. 1PPBCh. 16.13 - Practice Problem CONCEPTUALIZE
Which of the...Ch. 16.14 - Practice Problem ATTEMPT
Calculate the of a weak...Ch. 16.14 - Practice Problem BUILD
Calculate the of a weak...Ch. 16.14 - Practice Problem CONCEPTUALIZE Calculate K a...Ch. 16.15 - Practice Problem ATTEMPT
Calculate the pH at of a...Ch. 16.15 - Practice ProblemBUILD Calculate the pH at 25°C of...Ch. 16.15 - Practice Problem CONCEPTUALIZE
The diagrams...Ch. 16.16 - Practice ProblemATTEMPT Determine the K b of a...Ch. 16.16 - Practice Problem BUILD
Determine the of a weak...Ch. 16.16 - Practice Problem CONCEPTUALIZE
Determine the...Ch. 16.17 - Practice Problem ATTEMPT Determine (a) K b of the...Ch. 16.17 - Practice ProblemBUILD Determine (a) K b of the...Ch. 16.17 - Practice problemCONCEPTUALIZE Fee each week acid...Ch. 16.18 - Practice Problem ATTEMPT
Calculate the...Ch. 16.18 - Practice Problem BUILD
Calculate the...Ch. 16.18 - Practice ProblemCONCEPTURALIZE Which of the...Ch. 16.19 - Practice ProblemATTEMPT Indicate which is the...Ch. 16.19 - Practice Problem BUILD
Based on the information in...Ch. 16.19 - Prob. 1PPCCh. 16.20 - Practice ProblemATTEMPT Determine the pH of a...Ch. 16.20 - Practice ProblemBUILD Determine the concentration...Ch. 16.20 - Practice ProblemCONCEPTUALIZE Which of the...Ch. 16.21 - Practice ProblemATTEMPT Determine the pH of a...Ch. 16.21 - Practice ProblemBUILD Determine the concentration...Ch. 16.21 - Practice Problem CONCEPTUALIZE
Which of the...Ch. 16.22 - Practice Problem ATTEMPT
Predict whether a 0.10-M...Ch. 16.22 - Prob. 1PPBCh. 16.22 - Prob. 1PPCCh. 16.23 - Practice ProblemATTEMPT Identify the Lewis acid...Ch. 16.23 - Practice Problem BUILD
Write formulas for the...Ch. 16.23 - Practice Problem CONCEPTUALIZE
Which of the...Ch. 16 - Calculate the pH of a solution that is 0.22 M in...Ch. 16 - 16.2 Determine pH at the equivalence point in the...Ch. 16 - Calculate the pH of a solution that is 0.22 M in...Ch. 16 - 16.4 Determine pH at the equivalence point in the...Ch. 16 - Define Brønsted acids and bases. Give an example...Ch. 16 - For a species to act as a Brønsted base, an atom...Ch. 16 - 16.3 Classify each of the following species as a...Ch. 16 - Identify the acid-base conjugate pairs in each of...Ch. 16 - 16.5 Write the formulas of the conjugate bases of...Ch. 16 - Write the formula for the conjugate acid of each...Ch. 16 - Which of the following could represent a Brønsted...Ch. 16 - 16.8 Oxalic acid has the following structure:
An...Ch. 16 - Rite the equilibrium expression for the...Ch. 16 - 16.10 In Section 15.3 we learned that when we...Ch. 16 - 16.11 The equilibrium constant for the...Ch. 16 - 16.12 Define the term amphoteric.
Ch. 16 - 16.13 Compare the magnitudes of in aqueous...Ch. 16 - Calculate the OH - concentration in an aqueous...Ch. 16 - 16.15 Calculate the concentration in an aqueous...Ch. 16 - The value of K w at 50°C is 5.48 × 10 − 14 ....Ch. 16 - The value of K w at 100°C is 5.1 × 3 10 − 13 ....Ch. 16 - Prob. 18QPCh. 16 - Prob. 19QPCh. 16 - Prob. 20QPCh. 16 - Prob. 21QPCh. 16 - Prob. 22QPCh. 16 - Calculate the concentration of OH- ions in a 1 .4...Ch. 16 - Prob. 24QPCh. 16 - 16.25 Calculate the pH of each of the following...Ch. 16 - Calculate the pH of each of the following...Ch. 16 - Prob. 27QPCh. 16 - Prob. 28QPCh. 16 - 16.29 The pOH of a solution is 9.40 at . Calculate...Ch. 16 - Prob. 30QPCh. 16 - Prob. 31QPCh. 16 - 16.32 A solution is made by dissolving 18.4 g of ...Ch. 16 - Prob. 33QPCh. 16 - Prob. 34QPCh. 16 - Prob. 35QPCh. 16 - Prob. 36QPCh. 16 - Prob. 37QPCh. 16 - Prob. 38QPCh. 16 - Prob. 39QPCh. 16 - 16.40 Calculate the concentration of in a...Ch. 16 - Calculate the concentration of HNO 3 in a solution...Ch. 16 - Prob. 42QPCh. 16 - Prob. 43QPCh. 16 - Prob. 44QPCh. 16 - Prob. 45QPCh. 16 - Explain what is meant by the strength of an acid.Ch. 16 - Prob. 47QPCh. 16 - Prob. 48QPCh. 16 - Why do we normally not quote K a values for strong...Ch. 16 - Which of the following solutions has the highest...Ch. 16 - Without referring to the text, write the formulas...Ch. 16 - In biological and medical applications, it is...Ch. 16 - 16.53 The for benzoic acid is Calculate the pH...Ch. 16 - The K a for hydrofluoric acid is 7.1 × 10 − 4 ....Ch. 16 - Calculate the pH of an aqueous solution at 25°C...Ch. 16 - Calculate the pH of an aqueous solution at 25°C...Ch. 16 - 16.57 Determine the percent ionization of the...Ch. 16 - Prob. 58QPCh. 16 - Prob. 59QPCh. 16 - Prob. 60QPCh. 16 - Calculate the K a of a weak acid if a 0.19 − M...Ch. 16 - Prob. 62QPCh. 16 - What is the original molarity of a solution of...Ch. 16 - What is the original molarity of a solution of a...Ch. 16 - 16.65 Which of the following statements are true...Ch. 16 - Prob. 66QPCh. 16 - Prob. 67QPCh. 16 - Compare the pH values for 0.10 − M solutions of...Ch. 16 - Which of the following has a higher pH: (a) 1 .0 M...Ch. 16 - Prob. 70QPCh. 16 - The pH of a 0.30-M solution of a weak base is...Ch. 16 - What is the original molarity of an aqueous...Ch. 16 - Prob. 73QPCh. 16 - Prob. 74QPCh. 16 - Prob. 75QPCh. 16 - Prob. 76QPCh. 16 - Prob. 77QPCh. 16 - Prob. 78QPCh. 16 - 16.79 Calculate for each of the following ions: ...Ch. 16 - Prob. 80QPCh. 16 - Prob. 81QPCh. 16 - Prob. 82QPCh. 16 - Prob. 83QPCh. 16 - Prob. 84QPCh. 16 - Compare the pH of a 0 .040 M HCl solution with...Ch. 16 - What are the concentrations of HSO 4, – SO 2– 4 ,...Ch. 16 - 16.87 Calculate the concentrations of
Ch. 16 - Calculate the pH at 25°C of a 0.25 − M aqueous...Ch. 16 - 16.89 Calculate the pH at of a aqueous solution...Ch. 16 - The first and second ionization constants of a...Ch. 16 - Prob. 91QPCh. 16 - Prob. 92QPCh. 16 - Prob. 93QPCh. 16 - Prob. 94QPCh. 16 - Prob. 95QPCh. 16 - Prob. 96QPCh. 16 - Prob. 97QPCh. 16 - Define salt hydrolysis. Categorize salts according...Ch. 16 - 16.99 Explain why small, highly charged metal ions...Ch. 16 - Al 3+ is not a Brønsted acid, but Al( H 2 O ) 6 3+...Ch. 16 - Specify which of the following salts will undergo...Ch. 16 - Prob. 102QPCh. 16 - Calculate the pH of a 0 .42 M NH 4 Cl solution . (...Ch. 16 - Prob. 104QPCh. 16 - Prob. 105QPCh. 16 - Prob. 106QPCh. 16 - 16.107 Predict whether the following solutions are...Ch. 16 - A certain salt, MX (containing the M + and X -...Ch. 16 - Prob. 109QPCh. 16 - Predict whether a solution containing the salt K 2...Ch. 16 - Prob. 111QPCh. 16 - Prob. 112QPCh. 16 - Prob. 113QPCh. 16 - Prob. 114QPCh. 16 - Prob. 115QPCh. 16 - Prob. 116QPCh. 16 - Prob. 117QPCh. 16 - Prob. 118QPCh. 16 - Prob. 119QPCh. 16 - Prob. 120QPCh. 16 - Prob. 121QPCh. 16 - Prob. 122QPCh. 16 - Prob. 123QPCh. 16 - Prob. 124QPCh. 16 - Identity the Lewis acid and the Lewis base in the...Ch. 16 - Predict the direction that predominates in this...Ch. 16 - Prob. 127APCh. 16 - Prob. 128APCh. 16 - Calculate the pH and percent ionization of a 0 .88...Ch. 16 - 16.130 Calculate the pH of a 0.20 M ammonium...Ch. 16 - Prob. 131APCh. 16 - Prob. 132APCh. 16 - 16.133 Like water, liquid ammonia undergoes...Ch. 16 - Prob. 134APCh. 16 - A solution contains a weak monoprotic acid HA and...Ch. 16 - Prob. 136APCh. 16 - Prob. 137APCh. 16 - Prob. 138APCh. 16 - Prob. 139APCh. 16 - A 10.0-g sample of white phosphorus was burned in...Ch. 16 - Prob. 141APCh. 16 - Prob. 142APCh. 16 - Prob. 143APCh. 16 - Prob. 144APCh. 16 - 16.145 Give an example of (a) a weak acid that...Ch. 16 - Prob. 146APCh. 16 - Prob. 147APCh. 16 - Prob. 148APCh. 16 - When chlorine reacts with water, the resulting...Ch. 16 - Prob. 150APCh. 16 - Calculate the pH of a 2 .00 M NH 4 CN solution.Ch. 16 - Calculate the concentrations of all species in a 0...Ch. 16 - Prob. 153APCh. 16 - 16.154 Calculate the concentrations of all the...Ch. 16 - Prob. 155APCh. 16 - Calculate the pH of a solution that is 1.00 M HCN...Ch. 16 - How many grams of NaCN would you need to dissolve...Ch. 16 - A solution of formic acid ( HCOOH ) has a pH of...Ch. 16 - Calculate the pH of a 1-L solution containing...Ch. 16 - 16.160 A 1.87-g sample of Mg reacts with 80.0 mL...Ch. 16 - Prob. 161APCh. 16 - Prob. 162APCh. 16 - A 0.400 M formic acid ( HCOOH ) solution freezes...Ch. 16 - Prob. 164APCh. 16 - Prob. 165APCh. 16 - Prob. 166APCh. 16 - 16.167 Both the amide ion and the nitride ion ...Ch. 16 - Determine whether each of the following statements...Ch. 16 - Prob. 169APCh. 16 - Prob. 170APCh. 16 - Prob. 171APCh. 16 - 16.172 A typical reaction between an antacid and...Ch. 16 - Prob. 173APCh. 16 - 16.174 Hemoglobin is a blood protein that is...Ch. 16 - Tooth enamel is largely hydroxyapatite [ Ca 3 ( PO...Ch. 16 - Prob. 176APCh. 16 - Prob. 177APCh. 16 - About half of the hydrochloric acid produced...Ch. 16 - Prob. 179APCh. 16 - Prob. 180APCh. 16 - Prob. 181APCh. 16 - (a) Use VSEPR to predict the geometry of the...Ch. 16 - The following questions are not based on a...Ch. 16 - The following questions are not based on a...Ch. 16 - The following questions are not based on a...Ch. 16 - The following questions are not based on a...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 20.44 The Diels-Alder reaction is not limited to making six-membered rings with only car- bon atoms. Predict the products of the following reactions that produce rings with atoms other than carbon in them. OCCH OCCH H (b) CH C(CH₂)s COOCH མ་ནས་བ (c) N=C H -0.X- (e) H C=N COOCHS + CH2=CHCH₂ →→arrow_forwardGiven the attached data, provide the drawing for the corresponding structure.arrow_forwardno Ai walkthroughsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning  Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER
Acid-Base Equilibrium; Author: Bozeman Science;https://www.youtube.com/watch?v=l5fk7HPmo5g;License: Standard YouTube License, CC-BY
Introduction to Titrimetric analysis; Author: Vidya-mitra;https://www.youtube.com/watch?v=uykGVfn9q24;License: Standard Youtube License