
Concept explainers
a.
Interpretation:
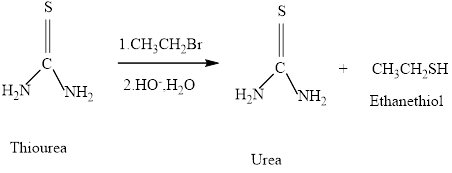
Thiols can be prepared from the reaction of thiourea with an
Concept introduction:
Organosulfur compound that contains a carbon –bonded sulfhydry group are called thiols. It has strong odour. The –SH froup is called a mercapto group. Named by adding the suffix –thiol to the
For example, the structure of 2-methyl propane 2-thiol.
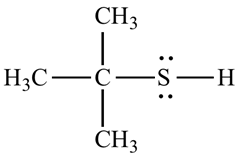
b.
Interpretation:
Thiols can be prepared from the reaction of thiourea with an alkyl halide , followed by hydroxide –io-promoted hydrolysis. What will be formed if the alkyl halide employed is pentyl bromide.
Concept introduction:
Organosulfur compound that contains a carbon –bonded sulfhydry group are called thiols. It has strong odour. The –SH froup is called a mercapto group. Named by adding the suffix –thiol to the alkane name. They are commonly prepared by an
For example, the structure of 2-methyl propane 2-thiol.
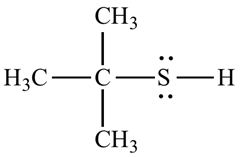
Want to see the full answer?
Check out a sample textbook solution
Chapter 16 Solutions
ORGANIC CHEMISTRY-W/S.G+SOLN.MANUAL
- Use curved arrows to generate a second resonance form for the allylic radical formed from 2-methyl-2-pentene. 1 Draw the curved arrows that would generate a second resonance form for this radical. D 2 H S F A Бг Iarrow_forwardDraw the resulting product(s) from the coupling of the given radicals. Inlcude all applicable electrons and non-zero formal charges. H.C öö- CH3 2nd attempt +1 : 招 H₂C CH CH₂ See Periodic Table See H H C S F P Br CH₂ Iarrow_forwardPlease, help me out with the calculation, step by step on how to find what's blank with the given information.arrow_forward
- Predict the following products. Then show the mechanism. H₂N NH2arrow_forwardBF3, Boron Trifluoride, known to contain three covalent boron-fluorine bonds. suggest and illustrate all of the processes as well as their energetical consequences for the formation of BF3 from its elements.arrow_forwardDraw the mechanism of the reaction.arrow_forward
- 9. Draw all of the possible Monochlorination Products that would Result From the Free Radical Chlormation OF 23,4-TRIMethyl Pentane b. Calculate the To Yield For the major • Product given the Following Relative Restritus For 1° 2° and 30 Hydrogens toward Free Radical Chloration 5.0: 38 : 1 30 2° 1° C. what would be the major product in the Free Radical brominator Of the Same Molecule. Explain your Reasoning.arrow_forwardWhat is the complete reaction mechanism for the chlorination of Ethane, C2H6?arrow_forwardA 13C NMR spectrum is shown for a molecule with the molecular formula of C6H100. Draw the structure that best fits this data. 220 200 180 160 140 120100 80 60 40 20 Drawingarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

