
(a)
Interpretation:
The product obtained from the given reaction should be identified.
Concept introduction:
Reactions of
Aldehydes and ketones reacts with conjugate base of peroxides to form
This reaction is called as Baeyer-Villiger oxidation.
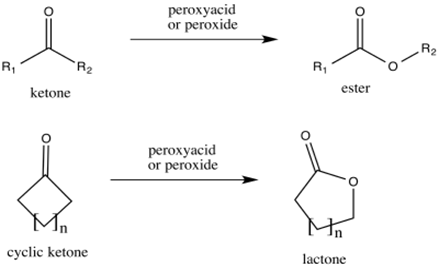
(b)
Interpretation:
The product obtained from the given reaction should be identified.
Concept introduction:
Reactions of aldehydes and ketones with peroxides:
Aldehydes and ketones reacts with conjugate bas of peroxides to form carboxylic acids and esters respectively.
This reaction is called as Baeyer-Villiger oxidation.
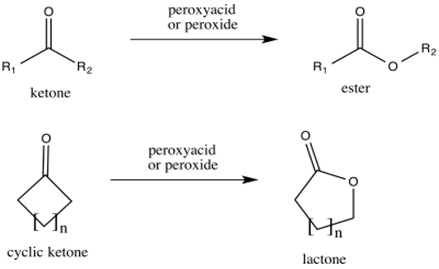
(c)
Interpretation:
The product obtained from the given reaction should be identified.
Concept introduction:
Reactions of aldehydes and ketones with peroxides:
Aldehydes and ketones reacts with conjugate bas of peroxides to form carboxylic acids and esters respectively.
This reaction is called as Baeyer-Villiger oxidation.
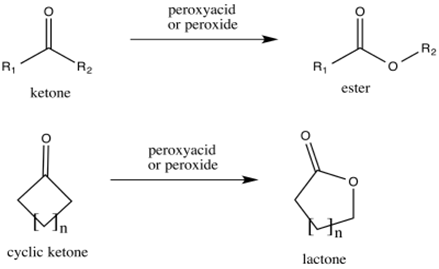
(d)
Interpretation:
The product obtained from the given reaction should be identified.
Concept introduction:
Reactions of aldehydes and ketones with peroxides:
Aldehydes and ketones reacts with conjugate bas of peroxides to form carboxylic acids and esters respectively.
This reaction is called as Baeyer-Villiger oxidation.
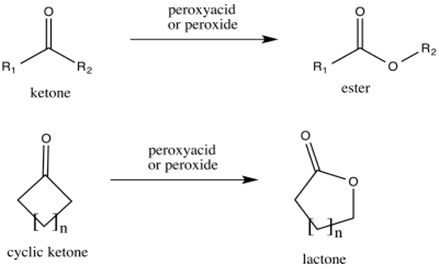
Want to see the full answer?
Check out a sample textbook solution
Chapter 16 Solutions
ORGANIC CHEMISTRY-W/S.G+SOLN.MANUAL
- Draw the major product of this reaction. Ignore inorganic byproducts. Assume that the water side product is continuously removed to drive the reaction toward products. (CH3)2NH, TSOH Drawingarrow_forwardSo, the first image is what I'm trying to understand regarding my approach. The second image illustrates my teacher's method, and the third image includes my notes on the concepts behind these types of problems.arrow_forwardHAND DRAWarrow_forward
- Draw a mental model for calcium chloride mixed with sodium phosphatearrow_forwardhere is my question (problem number 20) please explain to me thanks!arrow_forwardThe bromination of anisole is an extremely fast reaction. Complete the resonance structures of the intermediate arenium cation for the reaction (Part 1), and then answer the question that follows (Part 2).arrow_forward
- Drawing of 3-fluro-2methylphenolarrow_forwardWhich compound(s) will be fully deprotonated (>99%) by reaction with one molar equivalent of sodium hydroxide? I, II, III I, || I, III I only II, III SH | H3C-C=C-H || III NH2arrow_forwardWill NBS (and heat or light) work for this reaction, or do we have to use Br2?arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





