
a.
Interpretation:
Name the given compound.
Concept introduction:
The nomenclature of
Naming Aldehydes:
Aldehydes have at least one hydrogen attached to the carbonyl carbon atom.
The IUPAC naming of an aldehydes is obtained by replacing the final "e" on the name of the parent hydrocarbon with "al".
The common name of the aldehyde is substituted for "oic acid" at the end of parent hydrocarbon chain.
For example:
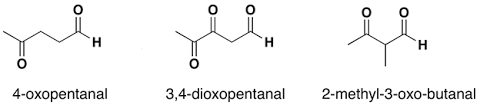
Naming Ketones:
Carbonyl carbon atom attached to the two oxygen atoms.
The IUPAC name of a ketones are obtained by replacing the "e" on the end of the parent hydrocarbon with "one".
Only few ketones have common name.
For example:
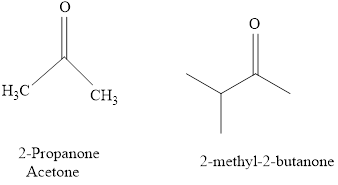
b.
Interpretation:
Name the given compound.
Concept introduction:
Naming Aldehydes:
Aldehydes have at least one hydrogen attached to the carbonyl carbon atom.
The IUPAC naming of an aldehydes is obtained by replacing the final "e" on the name of the parent hydrocarbon with "al".
The common name of the aldehyde is substituted for "oic acid" at the end of parent hydrocarbon chain.
For example:

Naming Ketones:
Carbonyl carbon atom attached to the two oxygen atoms.
The IUPAC name of a ketones are obtained by replacing the "e" on the end of the parent hydrocarbon with "one".
Only few ketones have common name.
For example:

c.
Interpretation:
Name the given compound.
Concept introduction:
Naming Aldehydes:
Aldehydes have at least one hydrogen attached to the carbonyl carbon atom.
The IUPAC naming of an aldehydes is obtained by replacing the final "e" on the name of the parent hydrocarbon with "al".
The common name of the aldehyde is substituted for "oic acid" at the end of parent hydrocarbon chain.
For example:

Naming Ketones:
Carbonyl carbon atom attached to the two oxygen atoms.
The IUPAC name of a ketones are obtained by replacing the "e" on the end of the parent hydrocarbon with "one".
Only few ketones have common name.
For example:

d.
Interpretation:
Name the given compound.
Concept introduction:
Naming Aldehydes:
Aldehydes have at least one hydrogen attached to the carbonyl carbon atom.
The IUPAC naming of an aldehydes is obtained by replacing the final "e" on the name of the parent hydrocarbon with "al".
The common name of the aldehyde is substituted for "oic acid" at the end of parent hydrocarbon chain.
For example:

Naming Ketones:
Carbonyl carbon atom attached to the two oxygen atoms.
The IUPAC name of a ketones are obtained by replacing the "e" on the end of the parent hydrocarbon with "one".
Only few ketones have common name.
For example:

Want to see the full answer?
Check out a sample textbook solution
Chapter 16 Solutions
ORGANIC CHEMISTRY-W/S.G+SOLN.MANUAL
- Question 3 What best describes the product of the following reaction? 1. CH3CH2MgBr (2 eq) 2. H a new stereocenter will not be formed a new stereocenter will be formed an alkyl halide will result an alkane will result an aromatic compound will result 1 ptsarrow_forwardRank the following from most to least reactive toward nucleophilic attack. 1. [Select] [Select] 2. Acyl halide Aldehyde 3. Carboxylate ion 4. Carboxylic acid Ketone 5. [Select]arrow_forwardQuestion 10 1 pts Which of the following is the most accurate nomenclature? 1-hydroxy-1-methyldecane-4,7-dione 2-hydroxy-2-methyldecane-5,8-dione 4,6-dioxo-2-methyldecane-2-ol 9-hydroxy-9-methyldecane-3,6-dione 8-hydroxy-8-methylnonane-3,6-dione OHarrow_forward
- Could you please explain whether my thinking is correct or incorrect regarding how I solved it? Please point out any mistakes in detail, with illustrations if needed.arrow_forwardWhat are the most proper reagents to achieve these products? سد 1. 2. OH ○ 1. BrMgC6H6; 2. H+ ○ 1. BrMgCH2CH2CH2CH2CH3; 2. H+ O 1. CH3CH2CHO; 2. H+ O 1. BrMgCH2CH3; 2. H+arrow_forwardProvide the IUPAC (systematic) name only for the following compound. Dashes, commas, and spaces must be correct. Harrow_forward
- Please use the nernst equation to genereate the Ion Selective Electrode Analysis standard curve within my excel spread sheet. Nernst Equation: E = Eo + m (ln a) Link: https://mnscu-my.sharepoint.com/:x:/g/personal/vi2163ss_go_minnstate_edu/EaREe1-PfGNKq1Cbink6kkYB5lBy05hEaE3mbGPUb22S6w?rtime=zQaSX3xY3Ugarrow_forwarda) b) c) H NaOH heat, dehydration + KOH heat, dehydration NaOH + (CH3)3CCHO heat, dehydration Pharrow_forwardshow mechanismarrow_forward
- Please draw by handarrow_forward3. Predict the major product and give a mechanism for the following reactions: (CH3)3COH/H₂SO4 a) b) NC CH₂O c) LOCH, (CH3)3COH/H2SO4 H,SO -OHarrow_forwardIndicate if the aldehyde shown reacts with the provided nucleophiles in acid or base conditions. a NaBH4 be Li eli -NH2 P(Ph3) f KCN g OH excess h CH3OH i NaCHCCH3arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning





