
Concept explainers
Show how each of the following compounds can be synthesized from cyclopentanol and any necessary organic or inorganic reagents. In many cases the desired compound can be made from one prepared in an earlier part of the problem.
trans-
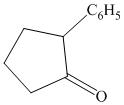
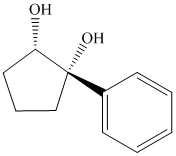
Interpretation:
Each of the given product is to be synthesized from cyclopentanol and necessary organic or inorganic reagents.
Concept introduction:
Alcohols can be prepared from a variety of reagents.
Reaction of Grignard reagents with carbonyl compounds produces the corresponding alcohols.
Grignard reagents also react with oxiranes to produce alcohols.
The allylic and benzylic carbon atoms are selectively brominated using NBS reagent.
Alcohols undergo dehydration in acidic medium, producing alkenes. These alkenes can be converted to diols using osmium tetra oxide.
Answer to Problem 23P
Solution:
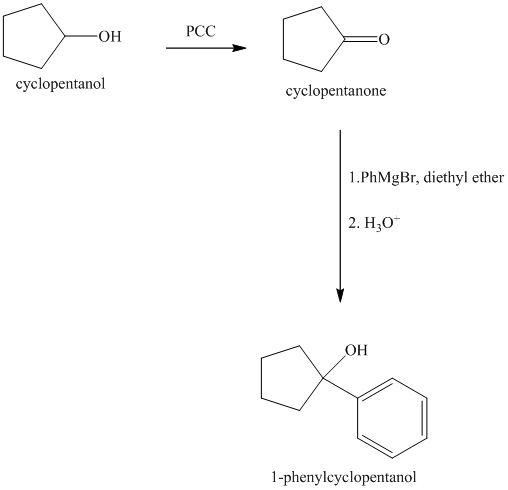
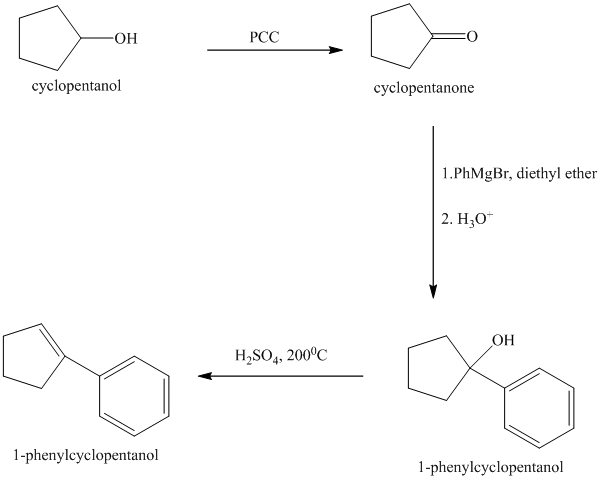
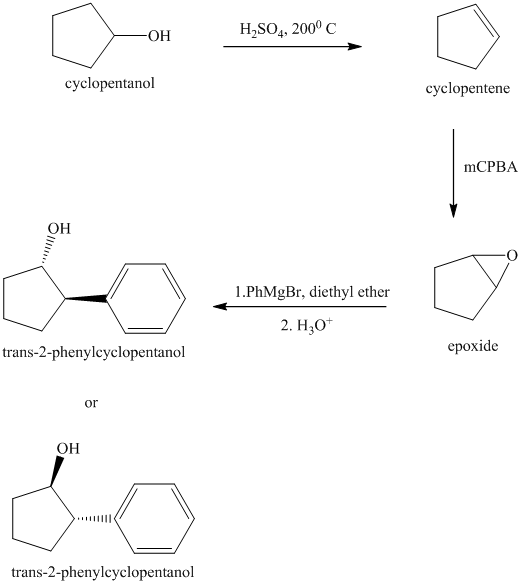
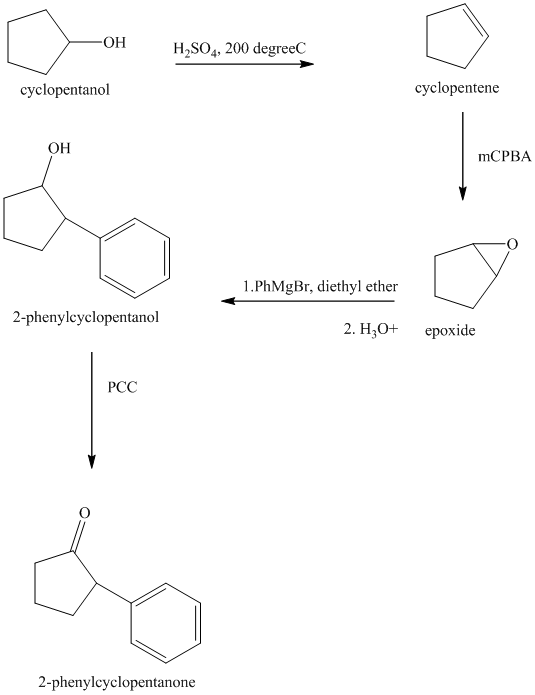
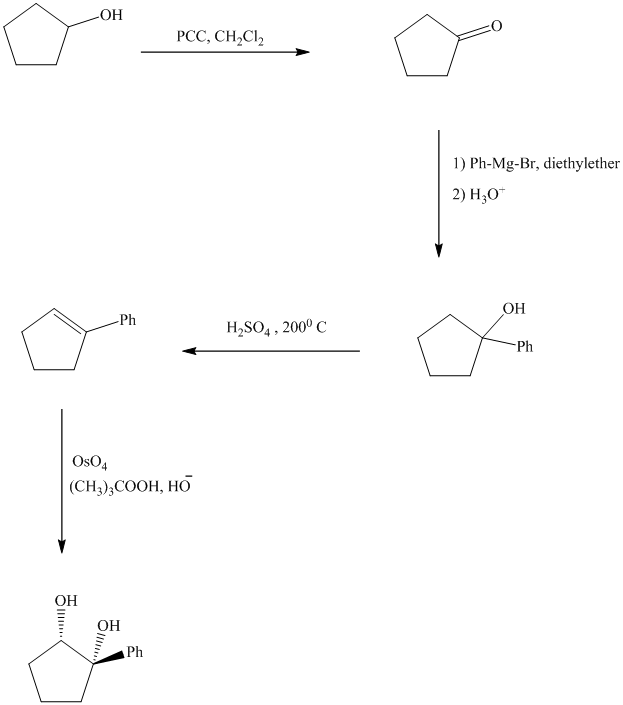
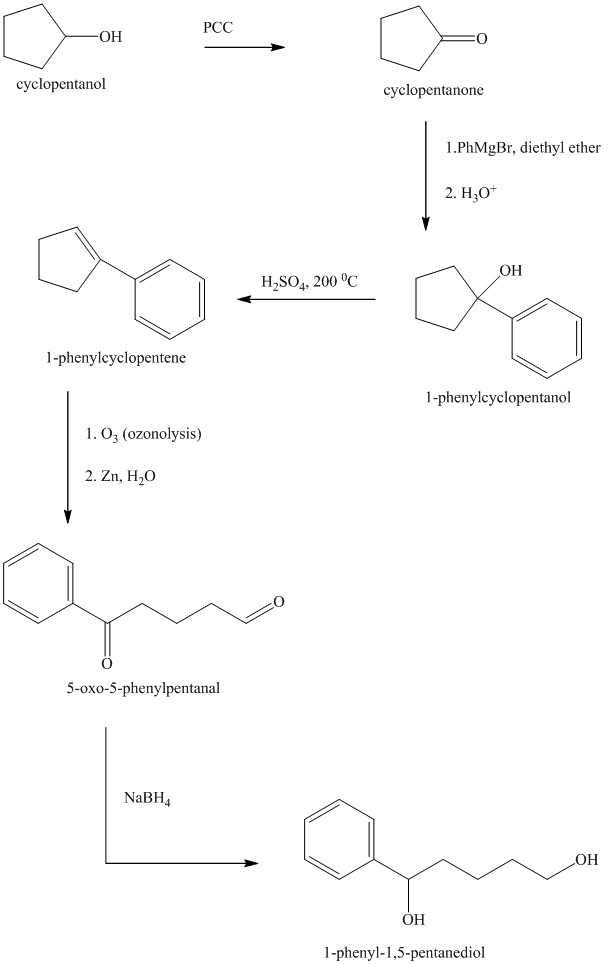
Explanation of Solution
Synthesis of
The structure for cyclopentanol and
In

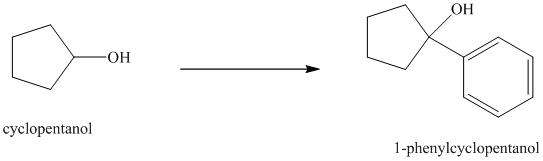
Synthesis of
The structure for cyclopentanol and
In
The sequence of reactions starting from cyclopentanol to yield the final given product

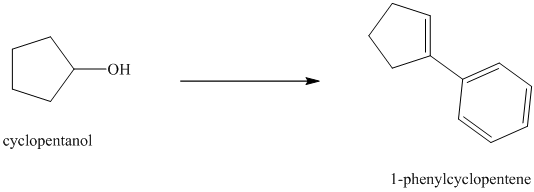
Synthesis of
The structure for cyclopentanol and
In
The sequence of reactions starting from cyclopentanol to yield the final given product

Synthesis of
The structure for cyclopentanol and
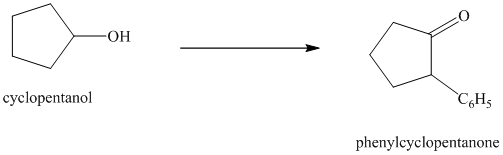
In
The sequence of reactions starting from cyclopentanol to yield the final given product
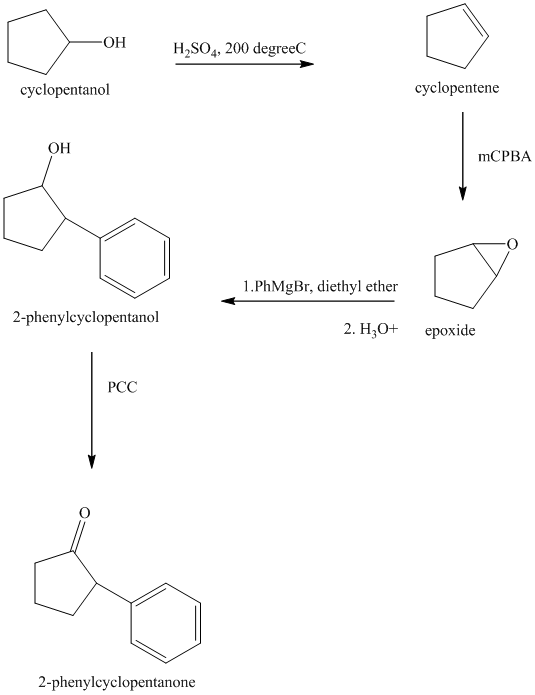
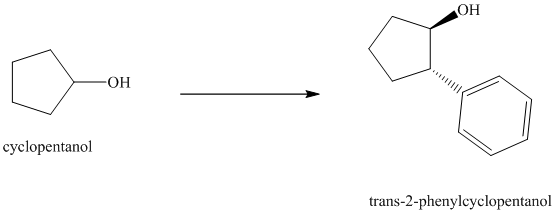
Synthesis of the given diol from cyclopentanol.
The structure for the given diol is as follows:
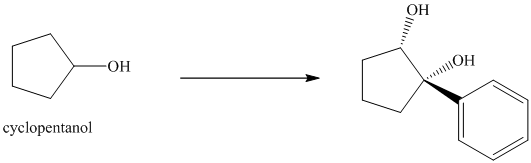
In the given diol, one phenyl ring and one hydroxyl group are attached to the same carbon of cyclopentane ring. The other hydroxyl group is attached to C2 position of cyclopentane ring.
Oxidation of the cyclopentanol will produce cyclopentanone. Reaction of this cyclopentanone with phenyl magnesium bromide will form a tertiary alcohol. Acid catalyzed dehydration of this tertiary alcohol will produce
The sequence of reactions starting from cyclopentanol to yield the final given product

Synthesis of
The structure for cyclopentanol and
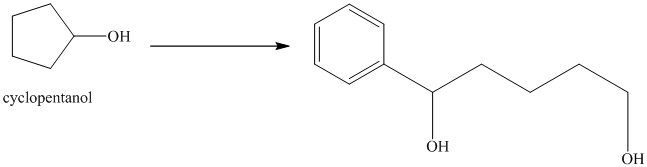
Cyclopentanol, when undergoes oxidation in presence of a mild oxidizing agent such as pyridinium chlorochromate in dichlormethane, the hydroxyl group turns to a carbonyl group and forms cyclopentanone. Cyclopentanone, when treated with Grignard reagent (PhMgBr) in the presence of diethyl ether with acidic workup, forms
The sequence of reactions is shown below.

Want to see more full solutions like this?
Chapter 16 Solutions
ORGANIC CHEMISTRY (LL)-W/SOLN.>CUSTOM<
- [In this question, there are multiple answers to type in a "fill-in-the-blank" fashion - in each case, type in a whole number.] Consider using Slater's Rules to calculate the shielding factor (S) for the last electron in silicon (Si). There will be electrons with a 0.35 S-multiplier, electrons with a 0.85 S-multiplier, and electrons with a 1.00 S-multiplier.arrow_forwardProvide the unknown for the given data.arrow_forwardDraw the Lewis structures of two methanol (CH3OH) molecules and depict hydrogenbonding between them with dashed lines. Show all lone pairs. Provide a thorough analysis to apply concept idea into other problems.arrow_forward
- Steps and explanation please.arrow_forwardHow could you distinguish between each pair of compounds below using IR? For each pair citeone bond and it’s frequency that you could use to distinguish between them. Please provide thorough analysis to apply into further problems.arrow_forwardSteps and explanation please.arrow_forward
- Provide the unknown for the given dataarrow_forwardProvide the unknown for the given data.arrow_forwardElectron Arrangement A. Fill in the following chart relating to levels, sublevels and orbitals. Levels (n) 1 Sublevels # of Orbitals per sublevel 2 3 4 # of Electrons per sublevel Total Electrons per level Complete: B. Answer the following questions related to levels, sublevels, orbitals and electrons. 1. How many sublevels are in energy level 2? 2. How many orbitals are in a 4f sublevel? 3. How many electrons can level 3 hold? 4. How many orbitals are in level 4? 5. How many electrons can sublevel 2p hold? 11arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

