
Concept explainers
(a)
Interpretation: The second resonance structure for given species and its hybrid is to be drawn. The two resonance structure and the hybrid are to be ranked in order of increasing stability.
Concept introduction: Most of the organic structures cannot be represented using single Lewis structure. Therefore, there exists more than one Lewis structure for representing a molecule or ion. These structures are known as resonance structures. These are the hypothetical structures and do not specify the exact structure. These resonance structure combine together to give resonance hybrid that is lower in energy and is the most stable structure.
The delocalization of electrons results in the formation resonance structure.
Answer to Problem 16.6P
The second resonance structure for given species and its hybrid are,
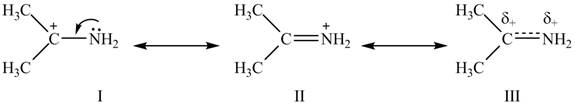
The increasing order of stability for the given resonance structure is
Explanation of Solution
The given species is shown below.
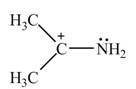
Figure 1
Delocalization of electrons from one position to the other position result in the formation of resonance structure. Resonance hybrid is the combination of all resonating structures. Thus, resonance structures and resonance hybrid for the given species is shown below.
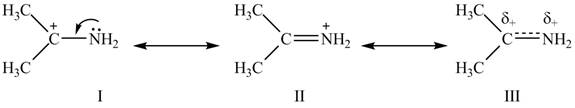
Figure 2
Better resonance structure is the one which has major contribution in the hybrid and others are minor contributors. Major contributor is the resonance structure which possesses more bonds and fewer charges.
The Figure 2 shows that the structure
Therefore, the increasing order of stability for the given resonance structure is
The second resonance structure for given species and its hybrid is shown in Figure 2. The increasing order of stability for the given resonance structure is is
(b)
Interpretation: The second resonance structure for given species and its hybrid is to be drawn. The two resonance structure and the hybrid are to be ranked in order of increasing stability.
Concept introduction: Most of the organic structures cannot be represented using single Lewis structure. Therefore, there exists more than one Lewis structure for representing a molecule or ion. These structures are known as resonance structures. These are the hypothetical structures and do not specify the exact structure. These resonance structure combine together to give resonance hybrid that is lower in energy and is the most stable structure.
The delocalization of electrons results in the formation resonance structure.
Answer to Problem 16.6P
The second resonance structure for given species and its hybrid are,
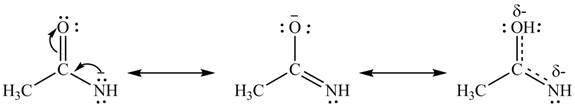
The increasing order of stability for the given resonance structure is
Explanation of Solution
The given species is shown below.
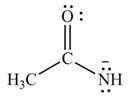
Figure 3
Delocalization of electrons from one position to the other position result in the formation of resonance structure. Resonance hybrid is the combination of all resonating structures. Thus, resonance structures and resonance hybrid for the given species is shown below.
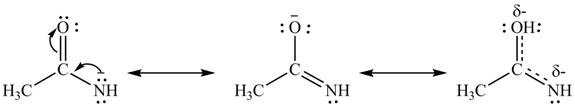
Figure 4
Better resonance structure is the one which has major contribution in the hybrid and others are minor contributors. Major contributor is the resonance structure in which negative charge is on more electronegative atom. The electronegativity of oxygen is more than nitrogen.
The Figure 4 shows that structure
Therefore, the increasing order of stability for the given resonance structure is
The second resonance structure for given species and its hybrid is shown in Figure 4. The increasing order of stability for the given resonance structure is is
(c)
Interpretation: The second resonance structure for given species and its hybrid is to be drawn. The two resonance structure and the hybrid are to be ranked in order of increasing stability.
Concept introduction: Most of the organic structures cannot be represented using single Lewis structure. Therefore, there exists more than one Lewis structure for representing a molecule or ion. These structures are known as resonance structures. These are the hypothetical structures and do not specify the exact structure. These resonance structure combine together to give resonance hybrid that is lower in energy and is the most stable structure.
The delocalization of electrons results in the formation resonance structure.
Answer to Problem 16.6P
The second resonance structure for given carbocation and its hybrid are,
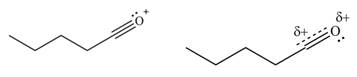
The increasing order of stability for the given resonance structure is,
Explanation of Solution
The given species is shown below.
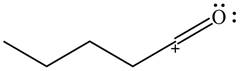
Figure 5
Delocalization of electrons from one position to the other position result in the formation of resonance structure. The resonance structure for the given species is shown below.
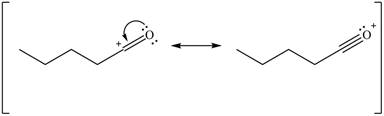
Figure 6
Resonance hybrid is the combination of all resonating structures. Thus, resonance hybrid for the given species is shown below.
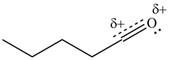
Figure 7
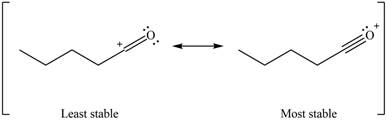
Better resonance structure is the one which has major contribution in the hybrid and others are minor contributors. Major contributor is the resonance structure which possesses more bonds and fewer charges.
Therefore, the increasing order of stability for the given resonance structure is shown below.

Figure 8
The second resonance structure for given carbocation and its hybrid is shown in Figure 6 and 7. The increasing order of stability for the given resonance structure is shown in Figure 8.
(d)
Interpretation: The second resonance structure for given species and its hybrid is to be drawn. The two resonance structure and the hybrid are to be ranked in order of increasing stability.
Concept introduction: Most of the organic structures cannot be represented using single Lewis structure. Therefore, there exists more than one Lewis structure for representing a molecule or ion. These structures are known as resonance structures. These are the hypothetical structures and do not specify the exact structure. These resonance structure combine together to give resonance hybrid that is lower in energy and is the most stable structure.
The delocalization of electrons results in the formation resonance structure.
Answer to Problem 16.6P
The second resonance structure for given carbocation and its hybrid are,
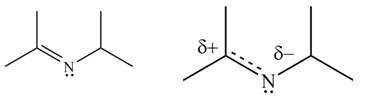
The increasing order of stability for the given resonance structure is,
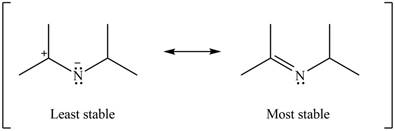
Explanation of Solution
The given species is shown below.
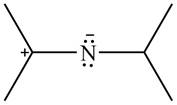
Figure 9
Delocalization of electrons from one position to the other position result in the formation of resonance structure. The resonance structure for the given species is shown below.
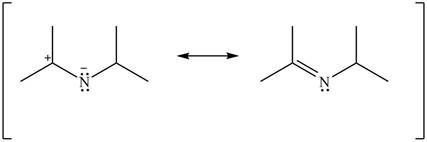
Figure 10
Resonance hybrid is the combination of all resonating structures. Thus, resonance hybrid for the given species is shown below.
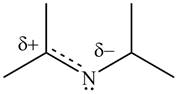
Figure 11
Better resonance structure is the one which has major contribution in the hybrid and others are minor contributors. Major contributor is the resonance structure which possesses more bonds and fewer charges.
The given Figure 2 shows that the left side structure does not contain double bond. Thus, the stability of left side structure is less than the right side structure.
Therefore, the increasing order of stability for the given resonance structure is shown below.
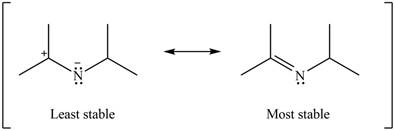
Figure 12
The second resonance structure for given carbocation and its hybrid is shown in Figure 10 and 11. The increasing order of stability for the given resonance structure is shown in Figure 12.
Want to see more full solutions like this?
Chapter 16 Solutions
Organic Chemistry-Package(Custom)
- Name an interesting derivative of barbituric acid, describing its structure.arrow_forwardBriefly describe the synthesis mechanism of barbituric acid from the condensation of urea with a β-diketone.arrow_forwardGiven the hydrazones indicated, draw the structures of the enamines that can be formed. Indicate the most stable enamine (explain). C6H5 C6H5 H C6H5 Harrow_forward
- 4. Propose a Synthesis for the molecule below. You may use any starting materials containing 6 carbons or less (reagents that aren't incorporated into the final molecule such as PhзP do not count towards this total, and the starting material can have whatever non-carbon functional groups you want), and any of the reactions you have learned so far in organic chemistry I, II, and III. Your final answer should show each step separately, with intermediates and conditions clearly drawn.arrow_forwardIndicate the importance of the indole ring. Find a representative example and list 5 structures.arrow_forwardΌΗ 1) V2 CO 3 or Nalt In منهarrow_forward
- 6. The equilibrium constant for the reaction 2 HBr (g) → H2(g) + Br2(g) Can be expressed by the empirical formula 11790 K In K-6.375 + 0.6415 In(T K-¹) - T Use this formula to determine A,H as a function of temperature. Calculate A,-H at 25 °C and at 100 °C.arrow_forward3. Nitrosyl chloride, NOCI, decomposes according to 2 NOCI (g) → 2 NO(g) + Cl2(g) Assuming that we start with no moles of NOCl (g) and no NO(g) or Cl2(g), derive an expression for Kp in terms of the equilibrium value of the extent of reaction, Seq, and the pressure, P. Given that K₂ = 2.00 × 10-4, calculate Seq/ of 29/no when P = 0.080 bar. What is the new value по ƒª/ at equilibrium when P = 0.160 bar? Is this result in accord with Le Châtelier's Principle?arrow_forwardConsider the following chemical equilibrium: 2SO2(g) + O2(g) = 2SO3(g) • Write the equilibrium constant expression for this reaction. Now compare it to the equilibrium constant expression for the related reaction: • . 1 SO2(g) + O2(g) = SO3(g) 2 How do these two equilibrium expressions differ? What important principle about the dependence of equilibrium constants on the stoichiometry of a reaction can you learn from this comparison?arrow_forward
- Given Kp for 2 reactions. Find the Kp for the following reaction: BrCl(g)+ 1/2 I2(g) ->IBr(g) + 1/2 Cl2(g)arrow_forwardFor a certain gas-phase reaction at constant pressure, the equilibrium constant Kp is observed to double when the temperature increases from 300 K to 400 K. Calculate the enthalpy change of the reaction, Ah, using this information.arrow_forwardHydrogen bonding in water plays a key role in its physical properties. Assume that the energy required to break a hydrogen bond is approximately 8 kJ/mol. Consider a simplified two-state model where a "formed" hydrogen bond is in the ground state and a "broken" bond is in the excited state. Using this model: • Calculate the fraction of broken hydrogen bonds at T = 300 K, and also at T = 273 K and T = 373 K. • At what temperature would approximately 50% of the hydrogen bonds be broken? • What does your result imply about the accuracy or limitations of the two-state model in describing hydrogen bonding in water? Finally, applying your understanding: • Would you expect it to be easier or harder to vaporize water at higher temperatures? Why? If you were to hang wet laundry outside, would it dry more quickly on a warm summer day or on a cold winter day, assuming humidity is constant?arrow_forward
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


