
Organic Chemistry-Package(Custom)
4th Edition
ISBN: 9781259141089
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 16, Problem 16.59P
A transannular Diels–Alder reaction is an intramolecular reaction that occurs when the diene and dienophile are contained in one ring, resulting in the formation of a tricyclic ring system. Draw the product formed when the following triene undergoes a transannular Diels–Alder reaction.
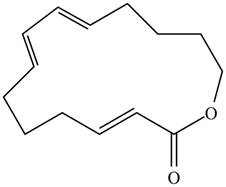
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Help w c!
Can someone help me understand this?
help w d!
Chapter 16 Solutions
Organic Chemistry-Package(Custom)
Ch. 16 - Prob. 16.1PCh. 16 - Prob. 16.2PCh. 16 - Draw a second resonance structure for each...Ch. 16 - Prob. 16.4PCh. 16 - Prob. 16.5PCh. 16 - Prob. 16.6PCh. 16 - Prob. 16.7PCh. 16 - Determine the hybridization of the labeled atom in...Ch. 16 - Problem 16.10 Draw the structure consistent with...Ch. 16 - Problem 16.11 Neuroprotectin D1 (NPD1) is...
Ch. 16 - Problem 16.12 Using hybridization, predict how the...Ch. 16 - Problem 16.13 Use resonance theory to explain why...Ch. 16 - Prob. 16.13PCh. 16 - Prob. 16.14PCh. 16 - Prob. 16.15PCh. 16 - Problem 16.17 Draw a stepwise mechanism for the...Ch. 16 - Prob. 16.17PCh. 16 - Problem 16.19 Draw the product formed when each...Ch. 16 - Prob. 16.19PCh. 16 - Prob. 16.20PCh. 16 - Rank the following dienophiles in order of...Ch. 16 - Prob. 16.22PCh. 16 - Prob. 16.23PCh. 16 - Prob. 16.24PCh. 16 - Prob. 16.25PCh. 16 - Problem 16.27 Which compound in each pair absorbs...Ch. 16 - Prob. 16.27PCh. 16 - 16.29 Name each diene and state whether the...Ch. 16 - Prob. 16.29PCh. 16 - Prob. 16.30PCh. 16 - Prob. 16.31PCh. 16 - Prob. 16.32PCh. 16 - Prob. 16.33PCh. 16 - Prob. 16.34PCh. 16 - 16.35 Explain why the cyclopentadienide anion A...Ch. 16 - Explain each statement using resonance theory. a....Ch. 16 - 16.37 Draw the structure of each compound.
a. in...Ch. 16 - Draw and name all dienes of molecular formula...Ch. 16 - Prob. 16.39PCh. 16 - 16.39 Label each pair of compounds as...Ch. 16 - Prob. 16.41PCh. 16 - 16.41 Draw the products formed when each compound...Ch. 16 - Prob. 16.43PCh. 16 - 16.43 Treatment of alkenes A and B with gives the...Ch. 16 - 16.44 Draw a stepwise mechanism for the following...Ch. 16 - Prob. 16.46PCh. 16 - 16.46 Explain, with reference to the mechanism,...Ch. 16 - Prob. 16.48PCh. 16 - Prob. 16.49PCh. 16 - Prob. 16.50PCh. 16 - Prob. 16.51PCh. 16 - Prob. 16.52PCh. 16 - Prob. 16.53PCh. 16 - 16.53 Diels–Alder reaction of a monosubstituted...Ch. 16 - Prob. 16.55PCh. 16 - Prob. 16.56PCh. 16 - 16.55 Devise a stepwise synthesis of each compound...Ch. 16 - Prob. 16.58PCh. 16 - 16.57 A transannular Diels–Alder reaction is an...Ch. 16 - Draw a stepwise mechanism for the following...Ch. 16 - Draw the products of each reaction. Indicate the...Ch. 16 - Prob. 16.62PCh. 16 - Prob. 16.63PCh. 16 - Prob. 16.64PCh. 16 - 16.65 The treatment of isoprene with one...Ch. 16 - 16.66 The treatment of with forms B (molecular...Ch. 16 - Rank the following compounds in the order of...Ch. 16 - Prob. 16.68PCh. 16 - Prob. 16.69PCh. 16 - Prob. 16.70PCh. 16 - Prob. 16.71PCh. 16 - Prob. 16.72PCh. 16 - Prob. 16.73PCh. 16 - Prob. 16.74P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- a. Explain Why electron withdrawing groupe tend to be meta-Directors. Your answer Should lyclude all apropriate. Resonance contributing Structures 6. Explain why -ll is an ortho -pura drccton evon though chlorine has a very High Electronegativityarrow_forwardC. Ν Harrow_forwarda. H3C. N H3C CH3 HCNarrow_forward
- ол 2. восцапан (46:00) Curtius rearrangment 1. NaN3, heat -OHarrow_forwardQuestion 1. Please predict the products for each of the following reactions. Clearly show the regiochemistry (Markovnikov vs anti-Markovnikov) and stereochemistry (syn- vs anti- or both). If a mixture of enantiomers is formed, please draw all the enantiomers.arrow_forwardElectrochemistry. Briefly describe the Donnan potential.arrow_forward
- Indicate what the Luther equation is used for?arrow_forwardIndicate one aspect that benefits and another that makes it difficult to use the hydroquinone electrode to measure pH.arrow_forwardAt an electrified interface according to the Gouy-Chapman model, what types of interactions do NOT occur between the ions and the solvent according to this theory?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Alcohols, Ethers, and Epoxides: Crash Course Organic Chemistry #24; Author: Crash Course;https://www.youtube.com/watch?v=j04zMFwDeDU;License: Standard YouTube License, CC-BY