
Bundle: Introduction to General, Organic and Biochemistry, 11th + OWLv2, 4 terms (24 months) Printed Access Card
11th Edition
ISBN: 9781305705159
Author: Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 16, Problem 16.58P
16-58 Following is a structural formula of desosamine, a
sugar component of several macrolide antibiotics, including the erythromycins. The configuration shown here is that of the natural product. Erythromycin is produced by a strain of Streptomyces erythreus originally found in a soil sample from the Philippine Archipelago.
ch3
T
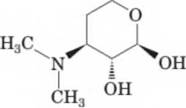
Desosamine
- Name all the
functional groups in desosamine. (Chapter 10) - How many stereocenters are present in desosamine? How many stereoisomers are possible for it? How many pairs of enantiomers are possible for it?
- Draw the alternative chair conformations for desosamine and label which groups are equatorial and which are axial.
(d > Which of the alternative chair conformations for desosamine is more stable?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Draw the skeletal ("line") structure of a secondary alcohol with 5 carbon atoms, 1 oxygen atom, at least one ring, and no double or triple bonds.
Click and drag to start drawing a
structure.
: ☐
☑
⑤
Name these organic compounds:
structure
name
CH₁₂
CH3 - C
CH
-
CH2
||
CH3-
-
CH₂
CH₂
|
-
-
CH3
CH3
2-methyl-2-butene
☐
3-methyl-1-butyne
-
CH3 CH.
-
C=CH
How many different molecules are drawn below?
Chapter 16 Solutions
Bundle: Introduction to General, Organic and Biochemistry, 11th + OWLv2, 4 terms (24 months) Printed Access Card
Ch. 16.1 - Problem 16-1 How many hydrogen atoms does...Ch. 16.2 - Problem 16-2 Write a structural formula for each...Ch. 16.2 - Prob. 16.3PCh. 16.4 - Problem 16-4 Select the stronger base from each...Ch. 16.5 - Prob. 16.5PCh. 16 - 16-6 Answer true or false. te/7-Butylamine is a 3°...Ch. 16 - Prob. 16.7PCh. 16 - Prob. 16.8PCh. 16 - 16-9 In what way are pyridine and pyrimidine...Ch. 16 - Prob. 16.10P
Ch. 16 - Prob. 16.11PCh. 16 - Prob. 16.12PCh. 16 - 16-13 Classify each amino group as primary,...Ch. 16 - Prob. 16.14PCh. 16 - 16-15 There are eight primary amines with the...Ch. 16 - Prob. 16.16PCh. 16 - 16-17 Propylamine (bp 48°C), ethylmethylamine (bp...Ch. 16 - 16-18 Account for the fact that 1-butanamine (bp...Ch. 16 - 16-19 2-Me thy 1 propane (bp -12°C), 2-propanol...Ch. 16 - Prob. 16.20PCh. 16 - Prob. 16.21PCh. 16 - Prob. 16.22PCh. 16 - Prob. 16.23PCh. 16 - Prob. 16.24PCh. 16 - Prob. 16.25PCh. 16 - 16-26 The p/fb of amphetamine is approximately 3.2...Ch. 16 - 16-27 Guanidine, p/Ca 13.6, is a very strong base,...Ch. 16 - 16-28 Following is the structural formula of...Ch. 16 - Prob. 16.29PCh. 16 - Prob. 16.30PCh. 16 - Prob. 16.31PCh. 16 - 16*32 Many tumors of the breast are correlated...Ch. 16 - Prob. 16.33PCh. 16 - Prob. 16.34PCh. 16 - 16-35 (Chemical Connections 16B ) What is an...Ch. 16 - Prob. 16.36PCh. 16 - Prob. 16.37PCh. 16 - Prob. 16.38PCh. 16 - Prob. 16.39PCh. 16 - Prob. 16.40PCh. 16 - Prob. 16.41PCh. 16 - Prob. 16.42PCh. 16 - Prob. 16.43PCh. 16 - Prob. 16.44PCh. 16 - Prob. 16.45PCh. 16 - 16-46 Arrange these three compounds in order of...Ch. 16 - Prob. 16.47PCh. 16 - Prob. 16.48PCh. 16 - Prob. 16.49PCh. 16 - Prob. 16.50PCh. 16 - Prob. 16.51PCh. 16 - Prob. 16.52PCh. 16 - Prob. 16.53PCh. 16 - 16-54 Several poisonous plants, including Atropa...Ch. 16 - Prob. 16.55PCh. 16 - Prob. 16.56PCh. 16 - Prob. 16.57PCh. 16 - 16-58 Following is a structural formula of...Ch. 16 - Prob. 16.59P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- With the reference to a anion A, Label compounds B-F as an isomer or resonance strcuture of A. FOr each isomer indicate what bonds differs from A. Provide steps and undertanding on how you come up with work.arrow_forwardProvide steps and also tips to undertand how to do on my own. Add the correct number of hydrogen atoms for each carbon atom and lone pairs to each oxygen atom.arrow_forwardA mixture of oxygen and ethyne is burnt for welding tell why mixture of ethyne and air is not usedarrow_forward
- Q2: Draw all applicable resonance forms for the acetate ion CH3COO. Clearly show all lone pairs, charges, and arrow formalism.arrow_forwardIn the following molecule, indicate the hybridization and shape of the indicated atoms. -z: CH3 CH 3 HO: H3C :Ö: CIarrow_forwardShow mechanism with explanation. don't give Ai generated solutionarrow_forward
- Please Help!!!arrow_forwardQ2: Resonance Forms a) Draw all resonance forms of the molecules. Include curved arrow notation. Label major resonance contributor. SO2 NO3 Page 3 of 4 Chem 0310 Organic Chemistry 1 HW Problem Sets CH3NSO (Thionitromethane, skeleton on the right) H N H3C Sarrow_forwardA 10.00-mL pipet was filled to the mark with distilled water at the lab temperature of 22 oC. The water, delivered to a tared weighing bottle was found to weigh 9.973 g. The density of water at 22 oC is 0.99780 g/mL. Calculate the volume of the pipet in mL. (disregard air displacement for this calculation and record your answer to the proper number of significant digits.)arrow_forward
- Resonance Formsa) Draw all resonance forms of the molecules. Include curved arrow notation. Label majorresonance contributor.arrow_forwardShow work with explanation needed. Don't give Ai generated solutionarrow_forwardf) The unusual molecule [2.2.2] propellane is pictured. 1) Given the bond length and bond angles in the image, what hybridization scheme best describes the carbons marked by the askerisks? 2) What types of orbitals are used in the bond between the two carbons marked by the askerisks? 3) How does this bond compare to an ordinary carbon-carbon bond (which is usually 1.54 Å long)? CH2 1.60Å H2C た C CH2 H2C H₂C * 120° C H2arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Acid-Base Titration | Acids, Bases & Alkalis | Chemistry | FuseSchool; Author: FuseSchool - Global Education;https://www.youtube.com/watch?v=yFqx6_Y6c2M;License: Standard YouTube License, CC-BY