
(a)
Interpretation:
The compounds mesitylene, toluene, and
Concept introduction:
The replacement of hydrogen atom attached to a carbon atom of electron-rich benzene ring by an electrophile is known as electrophilic aromatic substitution reaction. The rate of electrophilic aromatic substitution reaction depends on the substituted group on the aromatic ring. The ring deactivating group retards the electrophilic aromatic substitution reaction and ring activating group enhances the electrophilic aromatic substitution reaction.
Answer to Problem 16.44AP
The compounds mesitylene, toluene, and
Explanation of Solution
The structure of mesitylene, toluene, and
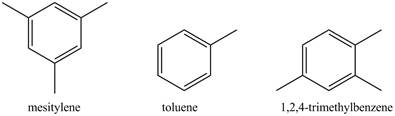
Figure 1
The reaction of any
The methyl group is electron-donating group. It activates the phenyl ring. The toluene has only one methyl group attached to it. Therefore, it will be least reactive towards
The order of reactivity toward nitration reaction is shown below.
The increasing order of reactivity towards toward
(b)
Interpretation:
The compounds chlorobenzene, benzene, and nitrobenzene are to be arranged in increasing order of increasing reactivity toward
Concept introduction:
The replacement of hydrogen atom attached to a carbon atom of electron-rich benzene ring by an electrophile is known as electrophilic aromatic substitution reaction. The rate of electrophilic aromatic substitution reaction depends on the substituted group on the aromatic ring. The ring deactivating group retards the electrophilic aromatic substitution reaction and ring activating group enhances the electrophilic aromatic substitution reaction.
Answer to Problem 16.44AP
The compounds chlorobenzene, benzene, and nitrobenzene are arranged in increasing order of increasing reactivity toward
Explanation of Solution
The structure of chlorobenzene, benzene, and nitrobenzene are shown below.
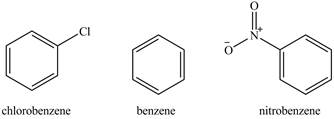
Figure 2
The reaction of any aromatic compound with
The nitro and chloro groups are electron-withdrawing groups. Therefore, the reactivity of the chlorobenzene and nitrobenzene will be less than that of benzene. The nitro group is stronger deactivating group than chloro group. Therefore, the order of reactivity toward nitration reaction is shown below.
The increasing order of reactivity towards toward
(c)
Interpretation:
The compounds
Concept introduction:
The replacement of hydrogen atom attached to a carbon atom of electron-rich benzene ring by an electrophile is known as electrophilic aromatic substitution reaction. The rate of electrophilic aromatic substitution reaction depends on the substituted group on the aromatic ring. The ring deactivating group retards the electrophilic aromatic substitution reaction and ring activating group enhances the electrophilic aromatic substitution reaction.
Answer to Problem 16.44AP
The compounds
Explanation of Solution
The structure of
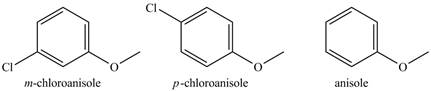
Figure 3
The reaction of any aromatic compound with
The methoxy group and chloro groups are ortho and para directing groups. The methoxy group is electron releasing group and chloro group is electron-withdrawing group.
Therefore, the reactivity of anisole will be highest among the rest of the compound toward
The order of reactivity toward nitration reaction is shown below.
The increasing order of reactivity towards toward
(d)
Interpretation:
The compounds acetophenone,
Concept introduction:
The replacement of hydrogen atom attached to a carbon atom of electron-rich benzene ring by an electrophile is known as electrophilic aromatic substitution reaction. The rate of electrophilic aromatic substitution reaction depends on the substituted group on the aromatic ring. The ring deactivating group retards the electrophilic aromatic substitution reaction and ring activating group enhances the electrophilic aromatic substitution reaction.
Answer to Problem 16.44AP
The compounds acetophenone,
Explanation of Solution
The structure of acetophenone,
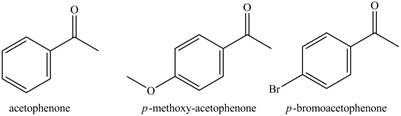
Figure 4
The reaction of any aromatic compound with
The acetyl group and bromo group is electron-withdrawing groups and methoxy group is electron releasing group. Acetophenone has a ring activating group attached on it. Therefore, it is most reactive toward nitration reaction among the rest of the compound. The compound
The increasing order of reactivity towards toward
Want to see more full solutions like this?
Chapter 16 Solutions
EBK ORGANIC CHEMISTRY STUDY GUIDE AND S
- Draw the Fischer projection from the skeletal structure shown below. HO OH OH OH OH H Q Drawing Atoms, Bonds and Rings Charges I ☐ T HO H H OH HO I CH2OH H OH Drag H OH -CH2OH CHO -COOH Undo Reset Remove Donearrow_forwardplease provide the structure for this problem, thank youarrow_forwardpresented by Morallen Lig Intermine the hand product for the given mution by adding atoms, bonds, nonhonding diarion panda скуль Step 3: Comp the draw the product Step 2: Agama workup Compithe 429 ملولةarrow_forward
- Reaction A 0,0arrow_forwardpresented by Morillon Leaning Predict the organic product for the min кусур HSC Adithane carved arnown to come than that to the condon slchroruis in acid in in aquishri with ноюarrow_forward6.15PM Sun Mar 30 K Draw the major product of this reaction. Include any relevant stereochemistry. Ignore inorganic byproducts. Problem 1 of O H [PhзPCH2CH3]*C|¯ NaH Drawing > Q Atoms, Bonds and Draw or tap a nearrow_forward
- 8:17 PM Sun Mar 30 Draw the major product of this reaction. Ignore inorganic byproducts. HSCH2CH2CH2SH, BF3 Probler Drawing Ato Bonds Clarrow_forwardpresented by Mr L How the coprion. (Il Done in no wraction, dew the starting redential) доarrow_forward8:16 PM Sun Mar 30 K Draw the major product of this reaction. Ignore inorganic byproducts. Proble 1. CH3MgBr 2. H3O+ F Drawingarrow_forward
- о но оarrow_forwardName the major organic product of the following action of 4-chloro-4-methyl-1-pentanol in neutral pollution 10+ Now the product. The product has a molecular formula f b. In a singly hain, the starting, material again converts into a secule with the molecular kormula CIO. but with comply Draw the major organic structure inhalationarrow_forwardMacmillan Learning Alcohols can be oxidized by chromic acid derivatives. One such reagent is pyridinium chlorochromate, (C,H,NH*)(CICTO3), commonly known as PCC. Draw the proposed (neutral) intermediate and the organic product in the oxidation of 1-butanol by PCC when carried out in an anhydrous solvent such as CH₂C₁₂. PCC Intermediate OH CH2Cl2 Draw the intermediate. Select Draw Templates More с H Cr о Product Draw the product. Erase Select Draw Templates More H о Erasearrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





