
LCPO CHEMISTRY W/MODIFIED MASTERING
8th Edition
ISBN: 9780135214756
Author: Robinson
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 16, Problem 16.41CP
Interpretation Introduction
Interpretation:
The name of picture of weakest diprotic acid
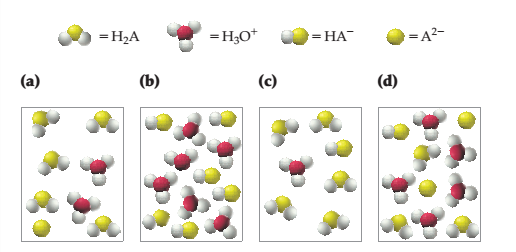
Concept introduction:
A chemical species that accepts electrons and donates protons or hydrogen ions is known as acid. In most acids, hydrogen atoms bonded that release to yield anion and cation in water.
The strength of the acid depends on the size and electronegativity of the atom directly attached to H atom.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Chapter 16 Solutions
LCPO CHEMISTRY W/MODIFIED MASTERING
Ch. 16 - Write a balanced equation for the dissociation of...Ch. 16 - Write the reaction between the carbonate ion...Ch. 16 - Conceptual PRACTICE 15.3 For the following...Ch. 16 - Conceptual APPLY 15.4 For the following reactions...Ch. 16 - If you mix equal concentrations of reactants and...Ch. 16 - Conceptual APPLY 15.6 The following pictures...Ch. 16 - Which pair has the stronger acid listed first? H2S...Ch. 16 - Which acid is stronger, H3PO4orH3AsO4?Ch. 16 - PRACTICE 15.9 The concentration of H3O+ ions in...Ch. 16 - Calculate the pH of a sample of seawater that has...
Ch. 16 - During mining operations, the mineral pyrite...Ch. 16 - Calculate the concentrations of H3O+ and OH- in a...Ch. 16 - Calculate the pH of the following solutions: (a)...Ch. 16 - Calculate the pH of a solution prepared by...Ch. 16 - The following pictures represent aqueous solutions...Ch. 16 - Acetic acid, CH3CO2H, is the solute that gives...Ch. 16 - Wha concentration of formic acid will result in a...Ch. 16 - Calculate the pH and the concentration of all...Ch. 16 - Carbonated drinks are prepared by dissolving CO2...Ch. 16 - Calculate the pH and the concentrations of all...Ch. 16 - Lactated Ringers solution is given intravenously...Ch. 16 - Prob. 16.25PCh. 16 - The following pictures represent aqueous solutions...Ch. 16 - Predict whether a solution of 0.20 M NaNO2 is...Ch. 16 - Calculate the pH and percent dissociation of...Ch. 16 - Prob. 16.29PCh. 16 - For the following Lewis acid— base reaction, draw...Ch. 16 - What are the chemical formulas and names of the...Ch. 16 - What were the average pH ranges for rainfall in...Ch. 16 - Prob. 16.33PCh. 16 - (a) Natural or “unpolluted” rain has a pH of 5.6....Ch. 16 - Prob. 16.35PCh. 16 - Prob. 16.36PCh. 16 - Because sulfur and nitrogen oxides are the main...Ch. 16 - Prob. 16.38CPCh. 16 - The following pictures represent aqueous solutions...Ch. 16 - Locate sulfur, selenium, chlorine, and bromine in...Ch. 16 - Prob. 16.41CPCh. 16 - Prob. 16.42CPCh. 16 - The followign pictures represent solutions of...Ch. 16 - Prob. 16.44CPCh. 16 - Look at the electron-dot structures of the...Ch. 16 - Boric acid (H3BO3) is a weak monoprotic acid that...Ch. 16 - Prob. 16.47CPCh. 16 - Prob. 16.48SPCh. 16 - Which of the following can behave both as a...Ch. 16 - Give the formula for the conjugate base of each of...Ch. 16 - Give the formula for the conjugate acid of each of...Ch. 16 - For each of the following reactions, identify the...Ch. 16 - For each of the following reactions, identify the...Ch. 16 - Aqueous solutions of hydrogen sulfide contain...Ch. 16 - Prob. 16.55SPCh. 16 - Choose from the conjugate acid-base pairs...Ch. 16 - Prob. 16.57SPCh. 16 - Prob. 16.58SPCh. 16 - Prob. 16.59SPCh. 16 - Arrange each group of compounds in order of...Ch. 16 - Arrange each group of compounds in order of...Ch. 16 - Prob. 16.62SPCh. 16 - Identify the weakest acid in each of the following...Ch. 16 - Prob. 16.64SPCh. 16 - Identify the stronger base in each of the...Ch. 16 - Prob. 16.66SPCh. 16 - Prob. 16.67SPCh. 16 - The concentration of OH- in a sample of seawater...Ch. 16 - The concentration of OH- in human blood is...Ch. 16 - For each of the following solutions, calculate...Ch. 16 - For each of the following solutions, calculate...Ch. 16 - Water superheated under pressure to 200oC and 750...Ch. 16 - Water at 500oC and 250 atm is a supercritical...Ch. 16 - Calculate the pH to the correct number of...Ch. 16 - Calculate the pH to the correct number of...Ch. 16 - Calculate the H3O+ concentration to the correct...Ch. 16 - Calculate the H3O+ concentration to the correct...Ch. 16 - Prob. 16.78SPCh. 16 - Which of the indicators given in Figure 16.5,...Ch. 16 - Which of the following species behave a strong...Ch. 16 - Which of the following species behave as strong...Ch. 16 - Calculate the pH of the following solutions:...Ch. 16 - Calculate the pH of the following solutions: 0.48...Ch. 16 - Prob. 16.84SPCh. 16 - Calculate the pH of solutions prepared by: RAN (a)...Ch. 16 - How many grams of CaO should be dissolved in...Ch. 16 - Prob. 16.87SPCh. 16 - Look up the value of Ka in Appendix C for...Ch. 16 - Look up the value of Ka in Appendix C for...Ch. 16 - The pH of 0.040 M hypobromous acid (HOBr) is 5.05....Ch. 16 - Lactic acid (C3H6O3) , which occurs in sour milk...Ch. 16 - The pH of 0.050 M gallic acid, an acid found in...Ch. 16 - The pH of 0.040 M pyruvic acid, an acid found in...Ch. 16 - A vitamin C tablet containing 250 mg of ascorbic...Ch. 16 - Acetic acid (CH3COOH;Ka=1.810-5) has a...Ch. 16 - Acrylic acid (HC3H3O2) is used in the manufacture...Ch. 16 - Hippuric acid (HC9H8NO3) , found in horse urine,...Ch. 16 - Calculat the pH and the percent dissociation in...Ch. 16 - A typical aspirin tablet contains 324 mg of...Ch. 16 - Prob. 16.100SPCh. 16 - Calculate the percent dissociation of...Ch. 16 - Write balanced net ionic equations and the...Ch. 16 - Write balanced net ionic equations and the...Ch. 16 - Calculate the pH and the concentrations of all...Ch. 16 - Prob. 16.105SPCh. 16 - Prob. 16.106SPCh. 16 - Tartaric acid (C4H6O6) is a diprotic acid that...Ch. 16 - Like sulfuric acid, selenic acid (H2SeO4) is a...Ch. 16 - Calculate the concentrations of H3O+ and SO42- in...Ch. 16 - Prob. 16.110SPCh. 16 - Prob. 16.111SPCh. 16 - Write a balanced net ionic equation and the...Ch. 16 - Write a balanced net ionic equation and the...Ch. 16 - Styrchine (C21H22N2O2) , a deadly poison used for...Ch. 16 - What is the pH of 0.5 M ammonia (NH3)?(Kb=1.8105)Ch. 16 - Morphine (C17H19NO3), a narcotic used in...Ch. 16 - A 1.00103M solution of quinine, a drug used in...Ch. 16 - Oxycodone (C18H21NO4), a narcotic analgesic, is a...Ch. 16 - Morpholine (C4H9NO) is a weak organic base with...Ch. 16 - Using values of Kb in Appendix C, calculate values...Ch. 16 - Using values of Ka in Appendix C, calculate values...Ch. 16 - Prob. 16.122SPCh. 16 - Sodium benzoate (C6H5CO2Na) is used as a food...Ch. 16 - Write a balanced net ionic equation for the...Ch. 16 - Write a balanced net ioflk equation for the...Ch. 16 - Classify each of the following ions according to...Ch. 16 - Classify each of the following salt solutions as...Ch. 16 - Calculate the concentrations of all species...Ch. 16 - Prob. 16.129SPCh. 16 - Calculate Ka for the cation Kb for the anion in an...Ch. 16 - Classify each of the following salt solutions as...Ch. 16 - Prob. 16.132SPCh. 16 - Classify each of the following salt solutions as...Ch. 16 - Calculate the pH and the concentrations of all...Ch. 16 - Calculate the pH and the percent dissociation of...Ch. 16 - Prob. 16.136SPCh. 16 - Prob. 16.137SPCh. 16 - Prob. 16.138SPCh. 16 - For each of the following reactions, identify the...Ch. 16 - Prob. 16.140SPCh. 16 - For each of the Lewis acid—base reactions in...Ch. 16 - Prob. 16.142SPCh. 16 - Prob. 16.143SPCh. 16 - Prob. 16.144MPCh. 16 - Prob. 16.145MPCh. 16 - Prob. 16.146MPCh. 16 - Prob. 16.147MPCh. 16 - Normal rain has a pH of 5.6 due to dissolved...Ch. 16 - Sulfur dioxide is quite soluble in water:...Ch. 16 - Prob. 16.150MPCh. 16 - Acid and base behavior can be observed in solvents...Ch. 16 - Prob. 16.152MPCh. 16 - In the case of very weak acids, [H3O+] from the...Ch. 16 - Prob. 16.154MPCh. 16 - Prob. 16.155MPCh. 16 - Neutralization reactions involving either a strong...Ch. 16 - Prob. 16.157MPCh. 16 - Prob. 16.158MPCh. 16 - A 200.0 mL sample of 0.350 M acetic acid (CH3CO2H)...Ch. 16 - Prob. 16.160MP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of the terms weak, strong, monoprotic, diprotic, and triprotic characterize(s) each of the following acids? More than one term may apply in a given situation. a. HC3H3O3 b. HCN c. H2SO4 d. H2SO3arrow_forwardArrange the following 0.10 M aqueous solutions in order of increasing pH: HF, NaF, HNO3, and NaNO3.arrow_forwardTo measure the relative strengths of bases stronger than OH, it is necessary to choose a solvent that is a weaker acid than water. One such solvent is liquid ammonia. (a) Write a chemical equation for the autoionization of ammonia. (b) What is the strongest acid and base that can exist in liquid ammonia? (c) Will a solution of HCI in liquid ammonia be a strong electrical conductor, a weak conductor, or a nonconductor? (d) Oxide ion (O2) is a stronger base than the amide ion (NH2). Write an equation for the reaction of O2 with NH3 in liquid ammonia. Will the equilibrium favor products or reactants?arrow_forward
- 8-13 Define (a) an Arrhenius acid and (b) an Arrhenius base.arrow_forwardWrite equations that show H2PO4- acting both as an acid and as a base.arrow_forwardStudents are often surprised to learn that organic acids, such as acetic acid, contain OH groups. Actually, all oxyacids contain hydroxyl groups. Sulfuric acid, usually written as H2SO4, has the structural formula SO2(OH)2, where S is the central atom. Identify the acids whose structural formulas are shown below. Why do they behave as acids, while NaOH and KOH are bases? a. SO(OH)2 b. ClO2(OH) c. HPO(OH)2arrow_forward
- In each of the following acid-base reactions, identify the Brnsted acid and base on the left and their conjugate partners on the right. (a) HCO2H(aq) + H2O() HCO2(aq) + H3O+(aq) (b) NH3(aq) + H2S(aq) NH4+(aq) + HS(aq) (c) HSO4(aq) + OH(aq) SO42(aq) + H2O+()arrow_forwardWhich of the following substances are acids in terms of the Arrhenius concept? Which are bases? Show the acid or base character by using chemical equations. a P4O10 b Na2O c N2H4 d H2Tearrow_forwardA Liquid HF undergoes an autoionization reaction: 2HFH2F++F (a) Is KF an acid or a base in this solvent? (b) Perchloric acid, HCIO4, is a strong acid in liquid HF. Write the chemical equation for the ionization reaction. (c) Ammonia is a strong base in this solvent. Write the chemical equation for the ionization reaction. (d) Write the net ionic equation for the neutralization of perchloric acid with ammonia in this solvent.arrow_forward
- Two strategies are also followed when solving for the pH of a base in water. What is the strategy for calculating the pH of a strong base in water? List the strong bases mentioned in the text that should be committed to memory. Why is calculating the pH of Ca(OH)2 solutions a little more difficult than calculating the pH of NaOH solutions? Most bases are weak bases. The presence of what element most commonly results in basic properties for an organic compound? What is present on this element in compounds that allows it to accept a proton? Table 13-3 and Appendix 5 of the text list Kb values for some weak bases. What strategy is used to solve for the pH of a weak base in water? What assumptions are made when solving for the pH of weak base solutions? If the 5% rule fails, how do you calculate the pH of a weak base in water?arrow_forwardAluminum chloride, AlCl3, reacts with trimethyl-amine, N(CH3)3. What would you guess to be the product of this reaction? Explain why you think so. Describe the reaction in terms of one of the acid base concepts. Write an appropriate equation to go with this description. Which substance is the acid according to this acidbase concept? Explain.arrow_forwardWrite chemical equations showing the individual proton-transfer steps that occur in aqueous solution for each of the following acids. a. H2CO3 (carbonic acid) b. H2C3H2O4 (malonic acid)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax
General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY