
To find:
In the titration of pyridine and
a) The pH at equivalence point.
b) The best choice indicator for this titration.
c) The pH for the titration after different volumes.
d) Sketch the titration curve for this titration and indicate which major species are present in the solution when pH = pKa at equivalence point and at the end of titration.
Answer to Problem 16.127QA
Solution:
a) pH at equivalence point =
b) Methyl orange
c) At
d)
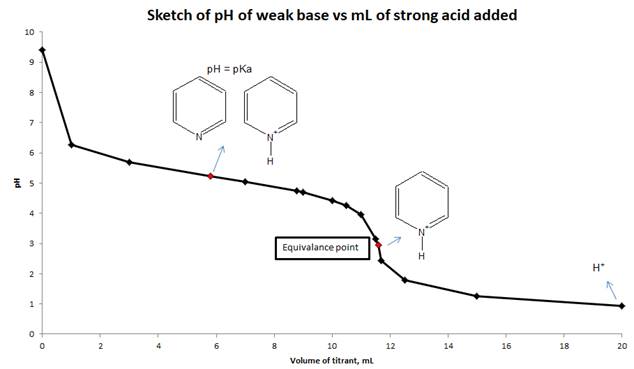
Explanation of Solution
1) Concept:
To calculate the pH of a weak base with a strong acid, we need to use reaction stoichiometry and RICE table. First, we will calculate the equivalence point from the volume and molarity of base and acid. We will calculate the pH at equivalence point by using RICE table. At equivalence point, moles of pyridine and HCl are the same, so the only species present is protonated pyridine. Using the hydrolysis of protonated pyridine and ICE table we can find the pH at the equivalence point. Suitable indicator for this titration is decided by comparing the pH at equivalence point and the pH range of indicators given in Figure 16.5. We will sketch the titration curve based on the pH of the solution and mL of a strong acid added to the weak base.
2) Formula:
i)
ii)
iii)
iv)
3) Given:
i) Concentration of pyridine =
ii) Volume of pyridine =
iii) Concentration of HCl =
iv)
v)
vi)
4) Calculations:
a) To calculate the equivalence point of the titration between pyridine and
From the balanced chemical equation, the mole ratio between pyridine and HCl is
At the equivalence point, moles of pyridine = moles of
Moles of
Volume of
The volume of
At equivalence point the number of moles of a weak base is equal to the number of moles of a strong acid. So, at the end of the reaction, the solution contains protonated pyridine which will govern the
| Initial | |||
| Added | |||
| Final | |||
We have the moles of the conjugate acid, hence, we can calculate the pH of the solution using the RICE table for protonated pyridine.
The dissociation reaction of protonated pyridine (conjugate acid) is written as:
Now calculating new molarity of conjugate acid as:
We can use RICE table to calculate the pH of the solution of conjugate acid.
| Initial | |||
| Change | |||
| Change | |||
Now write the
We will apply the 5% rule here, so we can ignore x from it,
The pH of the solution at equivalence point =
b) The pH of the solution at equivalence point =
c) Calculate the pH for the titration after following the volume of HCl added to a weak base.
1)
We can use RICE table to calculate the pH of the solution of pyridine at 0.0 mL addition of HCl
| Initial | |||
| Change | |||
| Equilibrium | |||
Now write the
We will apply the 5% rule here, so we can ignore x from it,
Initial pH of the pyridine =
2)
Calculate the moles of pyridine from 15.80 mL and 0.367 M.
Calculate the mole of HCl, when 1.0 mL added to the weak base.
We can use a modified RICE table to determine the how many moles of
| Initial | |||
| Change | |||
| Final | |||
The total sample volume is
Using these values in the Henderson–Hasselbalch equation gives us
3)
Calculate the mole of HCl, when
We can use a modified RICE table to determine the how many moles of
| Initial | |||
| Change | |||
| Final | |||
The total sample volume is
Using these values in the Henderson–Hasselbalch equation gives us
4)
Calculate the mole of HCl, when
We can use a modified RICE table to determine the how many moles of
| Initial | |||
| Change | |||
| Final | |||
The total sample volume is
Using these values in the Henderson–Hasselbalch equation gives us
5)
Calculate the mole of HCl, when
We can use a modified RICE table to determine the how many moles of
| Initial | |||
| Change | |||
| Final | |||
The total sample volume is
Using these values in the Henderson–Hasselbalch equation gives us
6)
Calculate the mole of HCl, when
We can use a modified RICE table to determine the how many moles of
| Initial | |||
| Change | |||
| Final | |||
The total sample volume is
Using these values in the Henderson–Hasselbalch equation gives us
7)
Calculate the mole of HCl, when
We can use a modified RICE table to determine the how many moles of
| Initial | |||
| Change | |||
| Final | |||
The total sample volume is
Using these values in the Henderson–Hasselbalch equation gives us
8)
Calculate the mole of HCl, when
We can use a modified RICE table to determine the how many moles of
| Initial | |||
| Change | |||
| Final | |||
The total sample volume is
Using these values in the Henderson–Hasselbalch equation gives us
9)
Calculate the mole of HCl, when
We can use a modified RICE table to determine the how many moles of
| Initial | |||
| Change | |||
| Final | |||
The total sample volume is
Using these values in the Henderson–Hasselbalch equation gives us
10)
Calculate the mole of HCl, when
We can use a modified RICE table to determine the how many moles of
| Initial | |||
| Change | |||
| Final | |||
HCl is a strong acid, the pH of solution can be calculated from the concentration of HCl.
The total sample volume is
11)
Calculate the mole of HCl, when
We can use a modified RICE table to determine the how many moles of
| Initial | |||
| Change | |||
| Final | |||
HCl is a strong acid, the pH of solution can be calculated from concentration of HCl.
The total sample volume is
12)
Calculate the mole of HCl, when
We can use a modified RICE table to determine the how many moles of
| Initial | |||
| Change | |||
| Final | |||
HCl is a strong acid, the pH of solution can be calculated from concentration of HCl.
The total sample volume is
13)
Calculate the mole of HCl, when
We can use a modified RICE table to determine the how many moles of
| Initial | |||
| Change | |||
| Final | |||
HCl is a strong acid, the pH of solution can be calculated from concentration of HCl.
The total sample volume is
d) Sketch the titration curve (pH vs mL of strong acid added) for this titration and show the major species are present in solution at pH = pka, equivalence point and end point.
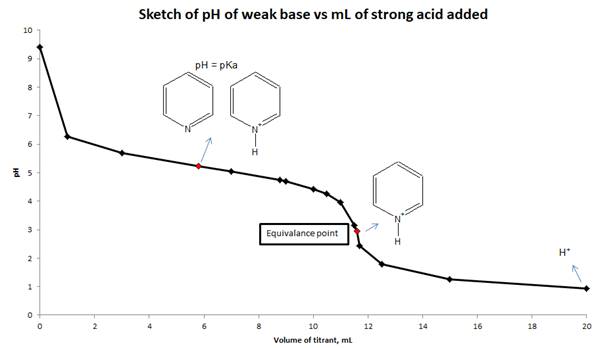
Conclusion:
The pH of the solution is calculated from reaction stoichiometry and RICE table at different mL of the titrant added to the weak base and also calculated at equivalence point. Sketch drawn on the basis of pH values and volume of strong acid added to a weak base.
Want to see more full solutions like this?
Chapter 16 Solutions
Chemistry: An Atoms-Focused Approach (Second Edition)
- In reactions whose kinetic equation is v = k[A]m, the rate coefficient k is always positive. Is this correct?arrow_forwardIf the concentration of A decreases exponentially with time, what is the rate equation? (A). -d[A] (B). dt d[A] = k[A] e-kt dtarrow_forwardGiven the first-order reaction: aA → products. State its kinetic equation.arrow_forward
- The following chemical structure represents a molecule of what molecular formula?arrow_forwardWhich region(s) of the following phospholipid is/are hydrophobic? RO I hydro-water phobic-dislikes = Hydrophobic dislikes water ○ I only Il only I and III only II and IV only O II, III, and IV only III || IVarrow_forwardPredict the product of the following reactions: O 0= excess Х Кон ОН H+ H+ Iarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





