
To sketch:
A titration curve for the titration of
a.
b.
c.
d.
Answer to Problem 16.46QA
Solution:
a)
b)
c)
d)
Explanation of Solution
1) Concept:
Oxalic acid is a weak diprotic acid, i.e., there are two protons that can dissociate from one molecule of oxalic acid in aqueous solution. The dissociation of protons occurs stepwise.
The first equivalence point occurs where the first dissociated proton is completely neutralized when titrated with a strong base
The second equivalence point occurs where both dissociated protons fully neutralized, and the equation is
Equation for complete titration is
At equivalence point, the equal moles of weak acid and strong base react and undergo neutralization. The second
2) Formula:
i)
ii)
iii)
iv)
v)
3) Given:
i) Molarity of oxalic acid,
ii) Volume of oxalic acid,
iii) Molarity of
iv)
v)
vi) pKa1 = 1.23 (Appendix 5 Table A5.1)
vii) pKa2 = 4.19 (Appendix 5 Table A5.1)
4) Calculation:
Converting volume in
Calculating moles of oxalic acid
These are the initial moles of oxalic acid present before any
Now, calculating the
a)
Converting volume in ml to L,
Calculating the moles of
Set up the RICE table that is used to calculate the moles of acid reacted and moles of conjugate base formed.
| Reaction | + | + | |||||
| Initial | 0 | ||||||
| Change | |||||||
| Final | 0 | ||||||
Calculating the concentration of
b)
Converting volume in ml to L,
Calculating the moles of
Setting up the RICE table to calculate the moles of acid reacted and moles of conjugate base formed,
| Reaction | + | + | |||||
| Initial | 0 | ||||||
| Change | |||||||
| Final | 0 | ||||||
Moles of the weak acid and conjugate base are equal.Thisis the half equivalence point to the first equivalence point, and the pH at half equivalence point is
c)
Converting volume in ml to L,
Calculating the moles of
Setting up the RICE that is used to calculate the moles of acid reacted and moles of conjugate base formed,
| Reaction | + | + | |||||
| Initial | 0 | ||||||
| Change | |||||||
| Final | 0 | ||||||
Calculating the concentration of
d)
Converting volume in ml to L,
Calculating the moles of
Set up the RICE table to calculate the moles of acid reacted and moles of conjugate base formed.
| Reaction | + | + | |||||
| Initial | 0 | ||||||
| Change | |||||||
| Final | 0 | ||||||
This is the first equivalence point since no moles of weak acid or strong base are left.
There is not much difference in the
At the equivalence point, the major species present is
Total volume of solution
Molarity of
| Initial (M) | |||
| Change (M) | |||
| Equilibrium (M) | |||
Writing the
A titration curve for the titration of
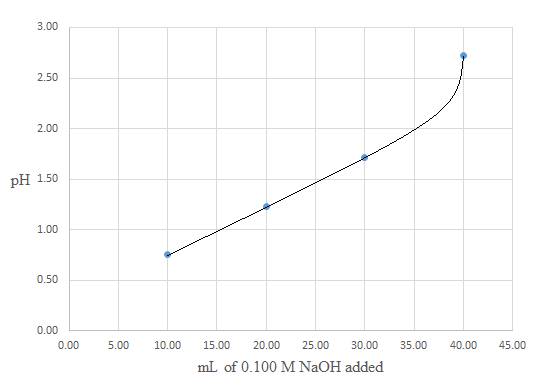
Conclusion:
Using the RICE table, we can determinethe
Want to see more full solutions like this?
Chapter 16 Solutions
Chemistry: An Atoms-Focused Approach (Second Edition)
- Can I get help on drawing my arrowsarrow_forwardCan I get helpp drawing my arrowsarrow_forwardWhich of the m/z values corresponds to the base peak in the mass spectrum shown? 100 80 A. 45 B. 44 C. 29 D. 15 Intensity 20 0 10 20 30 40 B- m/z -8 50 E. 30 Which of the m/z values correspond to the molecular ion for the compound shown? A. 18 B. 82 OH C. 100 D. 102 E. 103arrow_forward
- Please explain how to calculate the pH.arrow_forwardI'm having trouble with converting lewis diagrams into VSEPR diagrams. I currently have this example of C2BrCl3 which I want to turn into a lewis structure, but I'm not sure what steps I need to do in order to do so. I have the table written down, however, there's two central atoms so what would I do? There seems to be 4 electron domains on the carbon atom and no lone pairs so it would seem like this shape would be tetrahedral. Here's what I have now. Thanks!arrow_forwardWe discussed the solid phase resin using in peptide synthesis. Provide a mechanism, for its formation. DRAW THE MECHANISM.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





