
Concept explainers
Practice Problem 16.14
Dihydropyran reacts readily with an alcohol in the presence of a trace of anhydrous HCL or
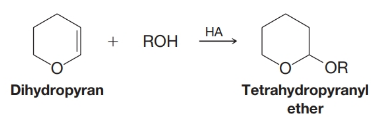
(a) Write a plausible mechanism for this reaction.
(b) Tetrahydropyranyl ethers are stable in aqueous base but hydrolyze rapidly in aqueous acid to yield the original alcohol and another compound. Explain. (What is the other compound?)
(c) The tetrahydropyranyl group can be used as a protecting group for alcohols and phenols. Show how you might use it in a synthesis of 5-methyl-1,5-hexanediol starting with 4-chloro-1-butanol.
Want to see the full answer?
Check out a sample textbook solution
Chapter 16 Solutions
ORGANIC CHEMISTRY (LL) W/WILEYPLUS NEXT
Additional Science Textbook Solutions
Fundamentals Of Thermodynamics
Living By Chemistry: First Edition Textbook
Human Anatomy & Physiology (Marieb, Human Anatomy & Physiology) Standalone Book
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Applications and Investigations in Earth Science (9th Edition)
Microbiology: An Introduction
- From the given compound, choose the proton that best fits each given description. a CH2 CH 2 Cl b с CH2 F Most shielded: (Choose one) Least shielded: (Choose one) Highest chemical shift: (Choose one) Lowest chemical shift: (Choose one) ×arrow_forwardConsider this molecule: How many H atoms are in this molecule? How many different signals could be found in its 1H NMR spectrum? Note: A multiplet is considered one signal.arrow_forwardFor each of the given mass spectrum data, identify whether the compound contains chlorine, bromine, or neither. Compound m/z of M* peak m/z of M + 2 peak ratio of M+ : M + 2 peak Which element is present? A 122 no M + 2 peak not applicable (Choose one) B 78 80 3:1 (Choose one) C 227 229 1:1 (Choose one)arrow_forward
 Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

