
ORGANIC CHEMISTRY (LL) W/WILEYPLUS NEXT
12th Edition
ISBN: 9781119664635
Author: Solomons
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 16, Problem 44P
Provide reagents that would accomplish each of the following syntheses. Begin by writing a retrosynthetic analysis.
(a)
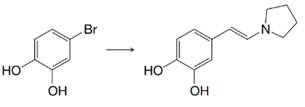
(b)
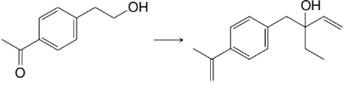
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
NMR spectrum of ethyl acetate has signals whose chemical shifts are indicated below. Which hydrogen or set of hydrogens corresponds to the signal at
4.1 ppm? Select the single best answer.
The
H
O
HỌC—C—0—CH, CH,
2
A
ethyl acetate
H NMR: 1.3 ppm, 2.0 ppm, 4.1 ppm
Check
OA
B
OC
ch
B
C
Save For Later
Submit Ass
© 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center |
How many signals do you expect in the H NMR spectrum for this molecule?
Br Br
Write the answer below.
Also, in each of the drawing areas below is a copy of the molecule, with Hs shown. In each copy, one of the H atoms is colored red. Highlight in red all other H
atoms that would contribute to the same signal as the H already highlighted red
Note for advanced students: In this question, any multiplet is counted as one signal.
1
Number of signals in the 'H NMR spectrum.
For the molecule in the top drawing area, highlight in red any other H atoms that will contribute to
the same signal as the H atom already highlighted red.
If no other H atoms will contribute, check the box at right.
Check
For the molecule in the bottom drawing area, highlight in red any other H atoms that will contribute
to the same signal as the H atom already highlighted red.
If no other H atoms will contribute, check the box at right.
O
✓
No additional Hs to color in top
molecule
ง
No additional Hs to color in bottom…
in the kinetics experiment, what were the values calculated? Select all that apply.a) equilibrium constantb) pHc) order of reactiond) rate contstant
Chapter 16 Solutions
ORGANIC CHEMISTRY (LL) W/WILEYPLUS NEXT
Ch. 16 - PRACTICE PROBLEM 16.1 (a) Give IUPAC substitutive...Ch. 16 - Prob. 2PPCh. 16 - Prob. 3PPCh. 16 - Practice Problem 16.4
Provide the reagents and...Ch. 16 - Prob. 5PPCh. 16 - Prob. 6PPCh. 16 - Prob. 7PPCh. 16 - Prob. 8PPCh. 16 - Prob. 9PPCh. 16 - Practice Problem 16.10
Shown below is the...
Ch. 16 - Prob. 11PPCh. 16 - Practice Problem 16.12
What product would be...Ch. 16 - Prob. 13PPCh. 16 - Practice Problem 16.14
Dihydropyran reacts readily...Ch. 16 - Practice Problem 16.15 Show how you might use...Ch. 16 - Practice Problem 16.16 (a) Show how you might...Ch. 16 - Practice Problem 16.17
In addition to...Ch. 16 - Practice Problem 16.18
Triphenylphosphine can be...Ch. 16 - Prob. 19PPCh. 16 - PRACTICE PROBLEM 16.20
Give the structure of the...Ch. 16 - PRACTICE PROBLEM 16.21 What would be the major...Ch. 16 - Prob. 22PCh. 16 - 16.23 Write structural formulas for the products...Ch. 16 - Give structural formulas for the products formed...Ch. 16 - 16.25 What products would be obtained when...Ch. 16 - Predict the major organic product from each of the...Ch. 16 - 16.27 Predict the major product from each of the...Ch. 16 - 16.28 Predict the major product from each of the...Ch. 16 - Prob. 29PCh. 16 - 16.30 Write detailed mechanisms for each of the...Ch. 16 - Prob. 31PCh. 16 - Prob. 32PCh. 16 - Show how you would convert benzaldehyde into each...Ch. 16 - 16.34 Show how ethyl phenyl ketone could be...Ch. 16 - Show how benzaldehyde could be synthesized from...Ch. 16 - Give structures for compounds AE. Cyclohexanol...Ch. 16 - Prob. 37PCh. 16 - Prob. 38PCh. 16 - Prob. 39PCh. 16 - Prob. 40PCh. 16 - Prob. 41PCh. 16 - Prob. 42PCh. 16 - 16.43 The structure of the sex pheromone...Ch. 16 - Provide reagents that would accomplish each of the...Ch. 16 - Write a detailed mechanism for the following...Ch. 16 - Prob. 46PCh. 16 - Dutch elm disease is caused by a fungus...Ch. 16 - Prob. 48PCh. 16 - Compounds W and X are isomers; they have the...Ch. 16 - Compounds Y and Z are isomers with the molecular...Ch. 16 - Compound A (C9H18O) forms a phenylhydrazone, but...Ch. 16 - Compound B (C8H12O2) shows a strong carbonyl...Ch. 16 - Prob. 53PCh. 16 - Prob. 54PCh. 16 - Prob. 55PCh. 16 - (a) What would be the frequencies of the two...Ch. 16 - Prob. 57PCh. 16 - Prob. LGP
Additional Science Textbook Solutions
Find more solutions based on key concepts
In what ways does connective tissue differ from epithelial tissue?
Principles of Anatomy and Physiology
21. Two shipwreck survivors were rescued from a life raft. One had drunk seawater while the other had not. The...
Introductory Chemistry (6th Edition)
Find the compressibility factor (Z) for saturated vapor ammonia at l00kPa and at 2000kPa .
Fundamentals Of Thermodynamics
7. Which bones form via intramembranous ossification?
a. Irregular bones
b. Certain flat bones
c. Long bones
d....
Human Anatomy & Physiology (2nd Edition)
How Would the experiments result charge if oxygen (O2) were induced in the spark chamber?
Biology: Life on Earth with Physiology (11th Edition)
Use the key to classify each of the following described tissue types into one of the four major tissue categori...
Anatomy & Physiology (6th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- true or false, given that a 20.00 mL sample of NaOH took 24.15 mL of 0.141 M HCI to reach the endpoint in a titration, the concentration of the NaOH is 1.17 M.arrow_forwardin the bromothymol blue experiment, pKa was measured. A closely related compound has a Ka of 2.10 x 10-5. What is the pKa?a) 7.1b) 4.7c) 2.0arrow_forwardcalculate the equilibrium concentration of H2 given that K= 0.017 at a constant temperature for this reaction. The inital concentration of HBr is 0.050 M.2HBr(g) ↔ H2(g) + Br2(g)a) 4.48 x 10-2 M b) 5.17 x 10-3 Mc) 1.03 x 10-2 Md) 1.70 x 10-2 Marrow_forward
- true or falsegiven these two equilibria with their equilibrium constants:H2(g) + CI2(l) ↔ 2HCI(g) K= 0.006 CI2(l) ↔ CI2(g) K= 0.30The equilibrium contstant for the following reaction is 1.8H2(g) + CI2 ↔ 2HCI(g)arrow_forwardI2(g) + CI2(g) ↔ 2ICIK for this reaction is 81.9. Find the equilibrium concentration of I2 if the inital concentration of I2 and CI2 are 0.010 Marrow_forwardtrue or false,the equilibrium constant for this reaction is 0.50.PCI5(g) ↔ PCI3(g) + CI2(g)Based on the above, the equilibrium constant for the following reaction is 0.25.2PCI5(g) ↔. 2PCI3(g) + 2CI2(g)arrow_forward
- true or false, using the following equilibrium, if carbon dioxide is added the equilibrium will shift toward the productsC(s) + CO2(g) ↔ 2CO(g)arrow_forward2S2O2/3- (aq) + I2 (aq) ---> S4O2/6- (aq) +2I- (aq) Experiment I2 (M) S2O3- (M) Initital Rate (M/s) 1 0.01 0.01 0.0004 2 0.01 0.02 0.0004 3 0.02 0.01 0.0008 Calculate the overall order for this reaction using the table data a) 3b) 0c) 2d) 1arrow_forwardthe decomposition of N2O5 is the first order with a half-life of 1.98 minutes. If the inital concentration of N2O5 is 0.200 M, what is the concentration after 6 minutes?a) 0.612 Mb) 0.035 Mc) 0.024 Md) 0.100 Marrow_forward
- 20.00 mL of 0.150 M HCI is titrated with 0.075 M NaOH. What volume of NaOH is needed?a) 50 mLb) 20 mLc) 40 mLd) 26.66 mLarrow_forward20.00 mL of 0.150 M NaOH is titrated with 37.75 mL of HCI. What is the molarity of the HCI?a) 0.150 Mb) 0.079 Mc) 0.025 Md) 0.050 Marrow_forwardin the following reaction, the OH- acts as which of these?NO2- (aq) + H2O (l) ⇌ OH- (aq) + HNO2 (aq)a) not a weak acidb) basec) acidarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY