
Concept explainers
(a)
Interpretation: The number of cannonballs needs to be determined that needs to stack by starting with a triangle in which each side is composed of 4 touching cannonballs.
Concept Introduction:
Solids are composed of certain repeating basic units which are called as unit cells. These units represent the arrangement of atoms in a certain manner that repeats itself throughout the solid and make the crystal lattice.
Each solid has a different type of unit cell and crystal lattice that makes it unique from other solids. The atoms are arranged in unit cells.
(b)
Interpretation: The type of arrangement that will be displayed by the cannonballs in the below arrangement needs to be explained.
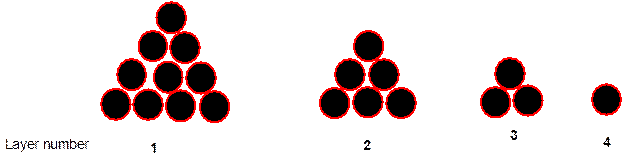
Concept Introduction:
Solids are composed of certain repeating basic units which are called as unit cells. These units represent the arrangement of atoms in a certain manner that repeats itself throughout the solid and make the crystal lattice.
Each solid has a different type of unit cell and crystal lattice that makes it unique from other solids. The atoms are arranged in unit cells.
(c)
Interpretation: The regular geometric solid in which 4 corners of the pyramid of cannonballs forms the corner needs to be determined.
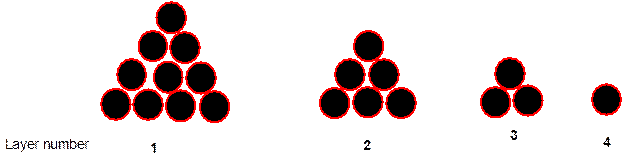
Concept Introduction:
Solids are composed of certain repeating basic units which are called as unit cells. These units represents the arrangement of atoms in a certain manner that repeats itself throughout the solid and make the crystal lattice.
Each solid has a different type of unit cell and crystal lattice that makes it unique from other solids. The atoms are arranged in unit cells.
Want to see the full answer?
Check out a sample textbook solution
Chapter 16 Solutions
EBK CHEMICAL PRINCIPLES
- A common prank on college campuses is to switch the salt and sugar on dining hall tables, which is usually easy because the substances look so much alike. Yet, despite the similarity in their appearance, these two substances differ greatly in their properties, since one is a molecular solid and the other is an ionic solid. How do the properties differ and why?arrow_forwardAn amorphous solid can sometimes be converted to a crystalline solid by a process called annealing. Annealing consists of heating the substance to a temperature just below the melting point of the crystalline form and then cooling it slowly. Explain why this process helps produce a crystalline solid.arrow_forward8.103 In previous chapters, we have noted that cryolite is used in refining aluminum. Use the web to look up what this addition does for the process, and relate it to the concepts of intermolecular forces from this chapter.arrow_forward
- The structures of some common crystalline substances are shown below. Show that the net composition of each unit cell corresponds to the correct formula of each substance.arrow_forwardConsider the CsCl unit shown in Figure 9.21. How many Cs+ ions are there per unit cell? How many Cl- ions? (Note that each Cl- ion is shared by eight cubes.)arrow_forwardSilicon carbide, SiC, is a very hard, high-melting solid. What kind of crystal forces account for these properties?arrow_forward
- What feature characterizes the dynamic equilibrium between a liquid and its vapor in a closed container?arrow_forwardConsider the following enthalpy changes: F+HFFHFH=155kJ/mol(CH3)2C=O+HF+(CH3)2CO---HFH=46kJ/molH2O(g)+HOH(g)+H2O---HOH(inice)H=21kJ/mol How do the strengths of hydrogen bonds vary with the electronegativity of the element to which hydrogen is bonded? Where in the preceding series would you expect hydrogen bonds of the following type to fall?arrow_forward8.76 Using circles, draw regular two-dimensional arrangements that demonstrate low packing efficiency and high packing efficieny.arrow_forward
- Why do liquids have a vapor pressure? Do all liquids have vapor pressures? Explain. Do solids exhibit vapor pressure? Explain. How does vapor pressure change with changing temperature? Explain.arrow_forward8.41 What is the specific feature of N, O, and F that causes them to play a role in hydrogen bonding?arrow_forwardHow do ionic solids differ in structure from molecular solids? What are the fundamental panicles in each? Give two examples of each type of solid and indicate the individual particles that make up the solids in each of your examples.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





