
THERMODYNAMICS (LL)-W/ACCESS >CUSTOM<
9th Edition
ISBN: 9781266657610
Author: CENGEL
Publisher: MCG CUSTOM
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 15.7, Problem 87P
Liquid octane (C8H18) enters a steady-flow combustion chamber at 25°C and 1 atm at a rate of 0.25 kg/min. It is burned with 50 percent excess air that also enters at 25°C and 1 atm. After combustion, the products are allowed to cool to 25°C. Assuming complete combustion and that all the H2O in the products is in liquid form, determine (a) the heat transfer rate from the combustion chamber, (b) the entropy generation rate, and (c) the exergy destruction rate. Assume that T0 = 298 K and the products leave the combustion chamber at 1 atm pressure.
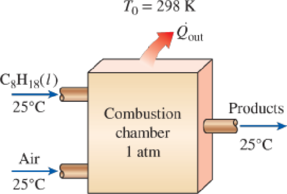
FIGURE P15–87
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
aversity of Baoyion
aculty of Engineering-AIMusyab
Automobile Eng. Dep.
Year: 2022-2023,
st Course, 1st Attempt
Stage: 3rd
Subject: Heat Transfer I
Date: 2023\01\23- Monday
Time: 3 Hours
Q4: A thick slab of copper initially at a uniform temperature of 20°C is suddenly exposed
to radiation at one surface such that the net heat flux is maintained at a constant value of
3×105 W/m². Using the explicit finite-difference techniques with a space increment of Ax
=
= 75 mm, determine the temperature at the irradiated surface and at an interior point that is
150 mm from the surface after 2 min have elapsed.
Q5:
(12.5 M)
A) A steel bar 2.5 cm square and 7.5 cm long is initially at a temperature of 250°C. It is
immersed in a tank of oil maintained at 30°C. The heat-transfer coefficient is 570 W/m². C.
Calculate the temperature in the center of the bar after 3 min.
B) Air at 90°C and atmospheric pressure flows over a horizontal flat plate at 60 m/s. The
plate is 60 cm square and is maintained at a…
University of Baby on
Faculty of Engineering-AIMusyab
Automobile Eng. Dep.
Year: 2022-2023.
1 Course, 1" Attempt
Stage 3
Subject Heat Transfer I
Date: 2023 01 23- Monday
Time: 3 Hours
Notes:
Q1:
•
•
Answer four questions only
Use Troles and Appendices
A) A flat wall is exposed to an environmental temperature of 38°C. The wall is covered
with a layer of insulation 2.5 cm thick whose thermal conductivity is 1.4 W/m. C,
and the temperature of the wall on the inside of the insulation is 315°C. The wall
loses heat to the environment by convection. Compute the value of the convection
heat-transfer coefficient that must be maintained on the outer surface of the
insulation to ensure that the outer-surface temperature does not exceed 41°C.
B) A vertical square plate, 30 cm on a side, is maintained at 50°C and exposed to room
air at 20°C. The surface emissivity is 0.8. Calculate the total heat lost by both sides
of the plate.
(12.5 M)
Q2: An aluminum fin 1.5 mm thick is placed on a circular tube…
Solve using graphical method and analytical method, only expert should solve
Chapter 15 Solutions
THERMODYNAMICS (LL)-W/ACCESS >CUSTOM<
Ch. 15.7 - What are the approximate chemical compositions of...Ch. 15.7 - How does the presence of N2 in air affect the...Ch. 15.7 - Prob. 3PCh. 15.7 - Prob. 4PCh. 15.7 - Is the airfuel ratio expressed on a mole basis...Ch. 15.7 - How does the presence of moisture in air affect...Ch. 15.7 - Prob. 7PCh. 15.7 - Prob. 8PCh. 15.7 - Prob. 9PCh. 15.7 - Are complete combustion and theoretical combustion...
Ch. 15.7 - What does 100 percent theoretical air represent?Ch. 15.7 - Consider a fuel that is burned with (a) 130...Ch. 15.7 - What are the causes of incomplete combustion?Ch. 15.7 - Which is more likely to be found in the products...Ch. 15.7 - Methane (CH4) is burned with the stoichiometric...Ch. 15.7 - Prob. 16PCh. 15.7 - n-Butane fuel (C4H10) is burned with the...Ch. 15.7 - Prob. 18PCh. 15.7 - Propane (C3H8) is burned with 75 percent excess...Ch. 15.7 - Propane fuel (C3H8) is burned with 30 percent...Ch. 15.7 - In a combustion chamber, ethane (C2H6) is burned...Ch. 15.7 - Prob. 22PCh. 15.7 - Prob. 23PCh. 15.7 - Ethane (C2H6) is burned with 20 percent excess air...Ch. 15.7 - Octane (C8H18) is burned with 250 percent...Ch. 15.7 - Prob. 26PCh. 15.7 - A fuel mixture of 60 percent by mass methane (CH4)...Ch. 15.7 - Prob. 28PCh. 15.7 - A certain natural gas has the following volumetric...Ch. 15.7 - Prob. 30PCh. 15.7 - A gaseous fuel with a volumetric analysis of 45...Ch. 15.7 - Prob. 33PCh. 15.7 - The fuel mixer in a natural gas burner mixes...Ch. 15.7 - Prob. 35PCh. 15.7 - Prob. 36PCh. 15.7 - Determine the fuelair ratio when coal from...Ch. 15.7 - Prob. 38PCh. 15.7 - Prob. 39PCh. 15.7 - Prob. 40PCh. 15.7 - Prob. 41PCh. 15.7 - When are the enthalpy of formation and the...Ch. 15.7 - Prob. 43PCh. 15.7 - Prob. 44PCh. 15.7 - Prob. 45PCh. 15.7 - Prob. 46PCh. 15.7 - Prob. 48PCh. 15.7 - Repeat Prob. 1546 for liquid octane (C8H18).Ch. 15.7 - Ethane (C2H6) is burned at atmospheric pressure...Ch. 15.7 - Reconsider Prob. 1550. What minimum pressure of...Ch. 15.7 - Calculate the HHV and LHV of gaseous n-octane fuel...Ch. 15.7 - Prob. 53PCh. 15.7 - Consider a complete combustion process during...Ch. 15.7 - Prob. 56PCh. 15.7 - Prob. 57PCh. 15.7 - Prob. 58PCh. 15.7 - Propane fuel (C3H8) is burned with an airfuel...Ch. 15.7 - Prob. 60PCh. 15.7 - Prob. 61PCh. 15.7 - Prob. 62PCh. 15.7 - Octane gas (C8H18) at 25C is burned steadily with...Ch. 15.7 - Liquid ethyl alcohol [C2H5OH(l)] at 25C is burned...Ch. 15.7 - Prob. 66PCh. 15.7 - A gaseous fuel mixture that is 40 percent propane...Ch. 15.7 - A constant-volume tank contains a mixture of 120 g...Ch. 15.7 - Prob. 70PCh. 15.7 - Prob. 71PCh. 15.7 - Prob. 72PCh. 15.7 - A fuel is completely burned first with the...Ch. 15.7 - Prob. 74PCh. 15.7 - Prob. 75PCh. 15.7 - What is the adiabatic flame temperature of methane...Ch. 15.7 - Octane gas (C8H18) at 25C is burned steadily with...Ch. 15.7 - Acetylene gas (C2H2) at 25C is burned during a...Ch. 15.7 - Ethyl alcohol [C2H5OH(g)] is burned with 200...Ch. 15.7 - Prob. 81PCh. 15.7 - Prob. 82PCh. 15.7 - Reconsider Prob. 1582. The combustion products are...Ch. 15.7 - Express the increase of entropy principle for...Ch. 15.7 - Prob. 85PCh. 15.7 - What does the Gibbs function of formation gf of a...Ch. 15.7 - Liquid octane (C8H18) enters a steady-flow...Ch. 15.7 - Prob. 88PCh. 15.7 - Reconsider Prob. 1588. The automobile engine is to...Ch. 15.7 - Benzene gas (C6H6) at 1 atm and 77F is burned...Ch. 15.7 - Prob. 91PCh. 15.7 - n-Octane [C8H18(l)] is burned in the...Ch. 15.7 - A steady-flow combustion chamber is supplied with...Ch. 15.7 - Prob. 94RPCh. 15.7 - Prob. 95RPCh. 15.7 - Prob. 96RPCh. 15.7 - Prob. 97RPCh. 15.7 - Prob. 98RPCh. 15.7 - Prob. 99RPCh. 15.7 - n-Butane (C4H10) is burned with the stoichiometric...Ch. 15.7 - A gaseous fuel mixture of 60 percent propane...Ch. 15.7 - Calculate the higher and lower heating values of...Ch. 15.7 - Prob. 103RPCh. 15.7 - Methane gas (CH4) at 25C is burned steadily with...Ch. 15.7 - A 6-m3 rigid tank initially contains a mixture of...Ch. 15.7 - Propane gas (C3H8) enters a steady-flow combustion...Ch. 15.7 - Determine the highest possible temperature that...Ch. 15.7 - Liquid propane [C3H8(l)] enters a combustion...Ch. 15.7 - Prob. 109RPCh. 15.7 - Prob. 110RPCh. 15.7 - Prob. 111RPCh. 15.7 - A steam boiler heats liquid water at 200C to...Ch. 15.7 - Repeat Prob. 15112 using a coal from Utah that has...Ch. 15.7 - Liquid octane (C8H18) enters a steady-flow...Ch. 15.7 - Prob. 115RPCh. 15.7 - Consider the combustion of a mixture of an...Ch. 15.7 - Prob. 117RPCh. 15.7 - A fuel is burned steadily in a combustion chamber....Ch. 15.7 - A fuel is burned with 70 percent theoretical air....Ch. 15.7 - Prob. 126FEPCh. 15.7 - One kmol of methane (CH4) is burned with an...Ch. 15.7 - The higher heating value of a hydrocarbon fuel...Ch. 15.7 - Acetylene gas (C2H2) is burned completely during a...Ch. 15.7 - An equimolar mixture of carbon dioxide and water...Ch. 15.7 - A fuel is burned during a steady-flow combustion...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- Need helparrow_forwardY F1 α В X F2 You and your friends are planning to move the log. The log. needs to be moved straight in the x-axis direction and it takes a combined force of 2.9 kN. You (F1) are able to exert 610 N at a = 32°. What magnitude (F2) and direction (B) do you needs your friends to pull? Your friends had to pull at: magnitude in Newton, F2 = direction in degrees, ẞ = N degarrow_forwardProblem 1 8 in. in. PROBLEM 15.109 Knowing that at the instant shown crank BC has a constant angular velocity of 45 rpm clockwise, determine the acceleration (a) of Point A, (b) of Point D. 8 in. Answer: convert rpm to rad/sec first. (a). -51.2j in/s²; (b). 176.6 i + 50.8 j in/s²arrow_forward
- Problem 4 The semicircular disk has a radius of 0.4 m. At one instant, when 0-60°, it is rotating counterclockwise at 0-4 rad/s, which is increasing in the same direction at 1 rad/s². Find the velocity and acceleration of point B at this instant. (Suggestion: Set up relative velocity and relative acceleration that way you would for a no-slip disk; remember what I told you to memorize on the first day of class.) (Answer: B = −2.98î - 0.8ĵ m/s, ãB = 2.45î - 5.74ĵ m/s²) B 0.4 m y Xarrow_forwardA C C 2r A 2r B B (a) (b) Problem 3 Refer to (b) of the figure shown above. The disk OA is now rolling with no slip at a constant angular velocity of w. Find the angular velocity and angular acceleration of link AB and BC. (Partial Answers: WBC = 2wk, AB = w²k)arrow_forwardProblem 2 Refer to (a) of the figure shown below, where the disk OA rotates at a constant angular velocity of w. Find the angular velocity and angular acceleration of link AB and link BC. (Partial Answers: WBC = wk, AB = w²k) A 2r C B (a) A 2r B (b)arrow_forward
- Example Two rotating rods are connected by slider block P. The rod attached at A rotates with a constant clockwise angular velocity WA. For the given data, determine for the position shown (a) the angular velocity of the rod attached at B, (b) the relative velocity of slider block P with respect to the rod on which it slides. b = 8 in., w₁ = 6 rad/s. Given: b = 8 in., WA = 6 rad/s CW constant Find: (a). WBE (b). Vp/Frame E 60° 20° Barrow_forwardY F1 α В X F2 You and your friends are planning to move the log. The log. needs to be moved straight in the x-axis direction and it takes a combined force of 2.9 kN. You (F1) are able to exert 610 N at a = 32°. What magnitude (F2) and direction (B) do you needs your friends to pull? Your friends had to pull at: magnitude in Newton, F2 = direction in degrees, ẞ = N degarrow_forward100 As a spring is heated, its spring constant decreases. Suppose the spring is heated and then cooled so that the spring constant at time t is k(t) = t sin + N/m. If the mass-spring system has mass m = 2 kg and a damping constant b = 1 N-sec/m with initial conditions x(0) = 6 m and x'(0) = -5 m/sec and it is subjected to the harmonic external force f (t) = 100 cos 3t N. Find at least the first four nonzero terms in a power series expansion about t = 0, i.e. Maclaurin series expansion, for the displacement: • Analytically (hand calculations) Creating Simulink Model Plot solutions for first two, three and four non-zero terms as well as the Simulink solution on the same graph for the first 15 sec. The graph must be fully formatted by code.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY
Extent of Reaction; Author: LearnChemE;https://www.youtube.com/watch?v=__stMf3OLP4;License: Standard Youtube License