
Masteringphysics With Pearson Etext - Valuepack Access Card - For College Physics
10th Edition
ISBN: 9780321976932
Author: YOUNG
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 15, Problem 9MCP
For the process shown in the pV diagram in Figure 15.28, the total work in going from a to d along the path shown is
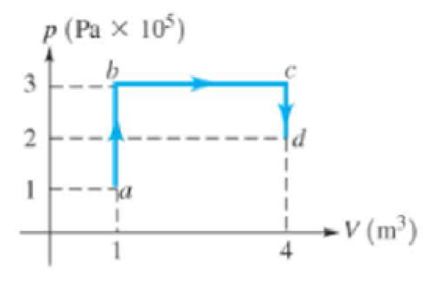
Figure 15.28
Multiple-Choice Problem 9
- A. 15 × 105 J
- B. 9 × 105 J.
- C. 6 × 105 J.
- D. 1 × 105 J.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
1. What is the spring constant of a spring that starts 10.0 cm long and extends to 11.4 cm with a 300 g mass hanging from it?
please help me solve all parts of this question from physics. thanks so much in advance! :)))
A fluid with density 263 kg/m3 flows through a pipe of varying diameter and height. At location 1 the flow speed is 13.5 m/s and the diameter of the pipe is 7.4 cm down to location 2 the pipe diameter is 16.9 cm. Location 1 is 6.3 meters higher than location 2.
What is the difference in pressure P2 - P1?
Using units in Pascals and use g = 9.81 m/s2.
Chapter 15 Solutions
Masteringphysics With Pearson Etext - Valuepack Access Card - For College Physics
Ch. 15 - In the ideal-gas equation could you give the...Ch. 15 - True or false? Equal masses of two different gases...Ch. 15 - How does evaporation of perspiration from your...Ch. 15 - The ideal-gas law is sometimes written in the form...Ch. 15 - (a) If you double the absolute temperature of an...Ch. 15 - Chemical reaction rates slow down as the...Ch. 15 - True or false? When two ideal gases are mixed,...Ch. 15 - Is it possible for a gas to expand and lose energy...Ch. 15 - The gas inside a balloon will always have a...Ch. 15 - When a gas expands adiabatically, it does work on...
Ch. 15 - Since Cv is defined with specific reference to a...Ch. 15 - The ratio y found in Equations 15.22 and 15.23...Ch. 15 - Prob. 1MCPCh. 15 - Prob. 2MCPCh. 15 - Prob. 3MCPCh. 15 - Prob. 4MCPCh. 15 - Prob. 5MCPCh. 15 - Prob. 6MCPCh. 15 - Assume you have n moles of an ideal gas initially...Ch. 15 - The formula U = nCvT for the change in the...Ch. 15 - For the process shown in the pV diagram in Figure...Ch. 15 - Prob. 10MCPCh. 15 - The gas shown in Figure 15.29 is in a completely...Ch. 15 - Prob. 12MCPCh. 15 - A cylindrical tank has a tight-fitting piston that...Ch. 15 - Prob. 2PCh. 15 - A 3.00 L tank contains air at 3.00 atm and 20.0C....Ch. 15 - A 20.0 L tank contains 0.225 kg of helium at...Ch. 15 - A room with dimensions 7.00 m by 8.00 m by 2.50 m...Ch. 15 - Three moles of an ideal gas are in a rigid cubical...Ch. 15 - A large cylindrical tank contains 0.750 m3 of...Ch. 15 - A 1.0 L canister contains 0.2 mole of helium gas....Ch. 15 - The gas inside a balloon will always have a...Ch. 15 - Prob. 10PCh. 15 - A diver observes a bubble of air rising from the...Ch. 15 - At an altitude of 11,000 m (a typical cruising...Ch. 15 - If a certain amount of ideal gas occupies a volume...Ch. 15 - Calculate the volume of 1.00 mol of liquid water...Ch. 15 - What volume does 2 mol of hydrogen gas (H2) occupy...Ch. 15 - The atmosphere of the planet Mars is 95.3% carbon...Ch. 15 - Find the mass of a single sulfur (S) atom and an...Ch. 15 - Prob. 18PCh. 15 - In the air we breathe at 72F and 1.0 atm pressure,...Ch. 15 - We have two equal-size boxes. A and B. Each box...Ch. 15 - Prob. 21PCh. 15 - Prob. 22PCh. 15 - A container of helium gas is heated until the...Ch. 15 - If 5 g of liquid helium is converted into a gas at...Ch. 15 - At what temperature is the root-mean-square speed...Ch. 15 - Where is the hydrogen? The average temperature of...Ch. 15 - Prob. 27PCh. 15 - STP. The conditions of standard temperature and...Ch. 15 - Prob. 29PCh. 15 - (a) How much heat does it take to increase the...Ch. 15 - (a) If you supply 1850 J of heat to 2.25 moles of...Ch. 15 - Suppose 100 J of heat flows into a diatomic ideal...Ch. 15 - Perfectly rigid containers each hold n moles of...Ch. 15 - Assume that the gases in this problem can be...Ch. 15 - A metal cylinder with rigid walls contains 2.50...Ch. 15 - A gas under a constant pressure of 1.50 105 Pa...Ch. 15 - Two moles of an ideal gas are heated at constant...Ch. 15 - Three moles of an ideal monatomic gas expand at a...Ch. 15 - Prob. 39PCh. 15 - Prob. 40PCh. 15 - A gas in a cylinder expands from a volume of 0.110...Ch. 15 - A gas in a cylinder is held at a constant pressure...Ch. 15 - Five moles of an ideal monatomic gas with an...Ch. 15 - When a system is taken from state a to state b in...Ch. 15 - An ideal gas expands while the pressure is Kept...Ch. 15 - You are keeping 1.75 moles of an ideal gas in a...Ch. 15 - Prob. 47PCh. 15 - A cylinder with a movable piston contains 3.00 mol...Ch. 15 - Figure 15.32 show a pV diagram for an ideal gas in...Ch. 15 - Figure 15.33 shows a pV diagram for an ideal gas...Ch. 15 - The pV diagram in Figure 15.34 shows a process abc...Ch. 15 - A volume of air (assumed to be an ideal gas) is...Ch. 15 - In the process illustrated by the pV diagram in...Ch. 15 - A cylinder contains 0.250 mol of carbon dioxide...Ch. 15 - Heating air in the lungs. Human lung capacity...Ch. 15 - The graph in Figure 15.37 shows a pV diagram for...Ch. 15 - An ideal gas at 4.00 atm and 350 K is permitted to...Ch. 15 - An experimenter adds 970 J of heat to 1.75 mol of...Ch. 15 - Heat Q flows into a monatomic ideal gas, and the...Ch. 15 - A player bounces a basketball on the floor,...Ch. 15 - In the pV diagram shown in Figure 15.38, 85.0 J of...Ch. 15 - Modern vacuum pumps make it easy to attain...Ch. 15 - Prob. 63GPCh. 15 - The effect of altitude on the lungs. (a) Calculate...Ch. 15 - (a) Calculate the mass of nitrogen present in a...Ch. 15 - An automobile tire has a volume of 0.0150 m3 on a...Ch. 15 - A student in a physics lab course has the task of...Ch. 15 - Prob. 68GPCh. 15 - Atmosphere of Titan. Titan, the largest satellite...Ch. 15 - Helium gas expands slowly to twice its original...Ch. 15 - A cylinder with a piston contains 0.250 mol of...Ch. 15 - You blow up a spherical balloon to a diameter of...Ch. 15 - A bicyclist uses a tire pump whose cylinder is...Ch. 15 - The bends. If deep-sea divers rise to the surface...Ch. 15 - 75. Figure 15.39 shows a pV diagram for 0.0040...Ch. 15 - Figure 15.40 Problem 76. The graph in Figure 15.40...Ch. 15 - A flask with a volume of 1.50 L, provided with a...Ch. 15 - Initially at a temperature of 80.0C, 0.28 m3 of...Ch. 15 - In a cylinder, 4.00 mol of helium initially at...Ch. 15 - Starting with 2.50 mol of N2 gas (assumed to be...Ch. 15 - Insulating windows. One way to improve insulation...Ch. 15 - Estimate the ratio of the thermal conductivity of...Ch. 15 - The rate of effusionthat is, the leakage of a gas...Ch. 15 - Prob. 84PPCh. 15 - In another test, the gas is put into a cylinder...Ch. 15 - You have a cylinder that contains 500 L of the gas...Ch. 15 - In a hospital, pure oxygen may be delivered at 50...
Additional Science Textbook Solutions
Find more solutions based on key concepts
What main advantage do microbial plastics have over synthetic plastics?
Brock Biology of Microorganisms (15th Edition)
Draw the mechanism for the hydroxide ion-catalyzed cleavage of fructose-l.6-bisphosphate.
Organic Chemistry (8th Edition)
Using the South Atlantic as an example, label the beginning of the normal polarity period C that began 2 millio...
Applications and Investigations in Earth Science (9th Edition)
Examine the following diagrams of cells from an organism with diploid number 2n = 6, and identify what stage of...
Genetic Analysis: An Integrated Approach (3rd Edition)
Some organizations are starting to envision a sustainable societyone in which each generation inherits sufficie...
Campbell Essential Biology (7th Edition)
How can the freezing of water crack boulders?
Campbell Biology in Focus (2nd Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- The kitchen had a temperature 46 degrees Fahrenheit and was converted it to Kelvin. What is the correct number for this temperature (46 F) on the Kelvin scale?arrow_forwardWater is traveling at a speed of 0.65 m/s through a pipe with a cross-section radius of 0.23 meters. The water enters a section of pipe that has a smaller radius, only 0.11 meters. What is the speed of the water traveling in this narrower section of pipe?arrow_forwardA particular water pipe has a radius of 0.28 meters. If the pipe is completely filled with water, moving with average velocity 0.45 m/s, what is the flow rate of water through the pipe with units of cubic meters of water per second?arrow_forward
- Water is flowing through a horizontal pipe with two segments. In one segment, the water flows at a speed v1 = 4.52 m/s. In the second segment the speed of the water is v2 = 2.38 m/s. Based on Bernoulli's Principle, what is the difference in pressure (P2 - P1) between the two segments? Assume that the density of the water is 997 kg/m3 and give your answer as the number of Pascals (i.e. N/m2).arrow_forwardWater from the faucet is supplied to the hose at a rate of 0.00057 m3/s. At what speed (number of meters per second) does the water exit the nozzle if the cross sectional area of the narrow nozzle is 2.1 x 10-6 m2?arrow_forwardJason Fruits/Indiana University Research Communications Silver/ silver oxide Zinc zinc/oxidearrow_forward
- Car P moves to the west with constant speed v0 along a straight road. Car Q starts from rest at instant 1, and moves to the west with increasing speed. At instant 5, car Q has speed w0 relative to the road (w0 < v0). Instants 1-5 are separated by equal time intervals. At instant 3, cars P and Q are adjacent to one another (i.e., they have the same position). In the reference frame o f the road, at instant 3 i s the speed o f car Q greater than, less than, or equal to the speed of car P? Explain.arrow_forwardCar P moves to the west with constant speed v0 along a straight road. Car Q starts from rest at instant 1, and moves to the west with increasing speed. At instant 5, car Q has speed w0 relative to the road (w0 < v0). Instants 1-5 are separated by equal time intervals.arrow_forwardCar P moves to the west with constant speed v0 along a straight road. Car Q starts from rest at instant 1, and moves to the west with increasing speed. At instant 5, car Q has speed w0 relative to the road (w0 < v0). Instants 1-5 are separated by equal time intervals. Sketch and label a vector diagram illustrating the Galilean transformation of velocities that relates velocity of car P relative to the road, velocity of car Q relative to road, and velocity of car Q relative to car P at instant 3. In the frame of car P, at instant 3 is car Q moving to the west, moving to the east, or at rest? Explain.arrow_forward
- Just 5 and 6 don't mind 7arrow_forwardIn an electron gun, electrons are accelerated through a region with an electric field of magnitude 1.5 × 104 N/C for a distance of 2.5 cm. If the electrons start from rest, how fast are they moving after traversing the gun?arrow_forwardPlease solve and answer this problem correctly please. Thank you!!arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781285737027
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers, Technology ...
Physics
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:OpenStax College
Thermodynamics: Crash Course Physics #23; Author: Crash Course;https://www.youtube.com/watch?v=4i1MUWJoI0U;License: Standard YouTube License, CC-BY