
Chemistry In Focus
7th Edition
ISBN: 9781337399692
Author: Tro, Nivaldo J.
Publisher: Cengage Learning,
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 15, Problem 3SC
Interpretation Introduction
Interpretation:
The structures of ethyl-2-methylbutanoate and civetone are to be examined and upon opening a bottle containing the mixture of these two, which one would dominate initial smell is to be determined.
Concept introduction:
Civetone is an unsaturated macrocyclic
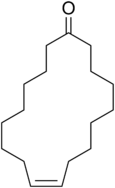
Civetone
Graham’s law states that the rate of diffusion is inversely proportional to the square root of mass of its particles.
Molar mass is defined as the ratio of moles
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Write the complete common (not IUPAC) name of each molecule below.
Note: if a molecule is one of a pair of enantiomers, be sure you start its name with D- or L- so we know which enantiomer it is.
molecule
Ο
C=O
common name
(not the IUPAC
name)
H
☐
H3N
CH₂OH
0-
C=O
H
NH3
CH₂SH
H3N
☐
☐
X
G
(Part A) Provide structures of the FGI products and missing reagents (dashed box)
1 eq Na* H*
H
-H
B1
B4
R1
H2 (gas)
Lindlar's
catalyst
A1
Br2
MeOH
H2 (gas)
Lindlar's
catalyst
MeO.
OMe
C6H1402
B2
B3
A1
Product carbons' origins
Draw a box around product
C's that came from A1.
Draw a dashed box around
product C's that came from B1.
Classify each of the amino acids below.
Note for advanced students: none of these amino acids are found in normal proteins.
X
CH2
H3N-CH-COOH3N-CH-COO-
H3N-CH-COO
CH2
CH3-C-CH3
CH2
NH3
N
NH
(Choose one) ▼
(Choose one)
S
CH2
OH
(Choose one) ▼
+
H3N-CH-COO¯
CH2
H3N CH COO H3N-CH-COO
CH2
오오
CH
CH3
CH2
+
O
C
CH3
O=
O_
(Choose one)
(Choose one) ▼
(Choose one)
G
Chapter 15 Solutions
Chemistry In Focus
Ch. 15 - Prob. 15.1YTCh. 15 - Prob. 1SCCh. 15 - Prob. 2SCCh. 15 - Prob. 3SCCh. 15 - Prob. 1ECh. 15 - Prob. 2ECh. 15 - Prob. 3ECh. 15 - Prob. 4ECh. 15 - Prob. 5ECh. 15 - Prob. 6E
Ch. 15 - Prob. 7ECh. 15 - Prob. 8ECh. 15 - Prob. 9ECh. 15 - Prob. 10ECh. 15 - Prob. 11ECh. 15 - Prob. 12ECh. 15 - Prob. 13ECh. 15 - Define eutrophication.Ch. 15 - Prob. 15ECh. 15 - Prob. 16ECh. 15 - Prob. 17ECh. 15 - Prob. 18ECh. 15 - Prob. 19ECh. 15 - What are the three types of interactions that...Ch. 15 - Prob. 21ECh. 15 - Prob. 22ECh. 15 - Prob. 23ECh. 15 - Prob. 24ECh. 15 - Prob. 25ECh. 15 - Prob. 26ECh. 15 - Prob. 27ECh. 15 - Prob. 28ECh. 15 - Prob. 29ECh. 15 - How do sunscreens protect your skin from the Suns...Ch. 15 - Prob. 31ECh. 15 - Prob. 32ECh. 15 - Prob. 33ECh. 15 - Prob. 34ECh. 15 - Prob. 35ECh. 15 - Prob. 36ECh. 15 - Prob. 37ECh. 15 - Prob. 38ECh. 15 - Prob. 39ECh. 15 - Prob. 40ECh. 15 - Prob. 41ECh. 15 - Prob. 42ECh. 15 - Prob. 43ECh. 15 - Prob. 44ECh. 15 - Prob. 45ECh. 15 - Prob. 46ECh. 15 - The salt bridges that hold hair protein (keratin)...Ch. 15 - Prob. 48ECh. 15 - The hydrochloric acid present in toilet bowl...Ch. 15 - Prob. 50ECh. 15 - Prob. 51ECh. 15 - Prob. 52ECh. 15 - Prob. 53ECh. 15 - Prob. 54ECh. 15 - Prob. 55ECh. 15 - Prob. 56ECh. 15 - Prob. 57ECh. 15 - Prob. 58ECh. 15 - Prob. 59ECh. 15 - Prob. 60ECh. 15 - Prob. 61ECh. 15 - Prob. 62ECh. 15 - Prob. 64ECh. 15 - Prob. 65E
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Another standard reference electrode is the standard calomel electrode: Hg2Cl2(s) (calomel) + 2e2 Hg() +2 Cl(aq) This electrode is usually constructed with saturated KCI to keep the Cl- concentration constant (similar to what we discussed with the Ag-AgCl electrode). Under these conditions the potential of this half-cell is 0.241 V. A measurement was taken by dipping a Cu wire and a saturated calomel electrode into a CuSO4 solution: saturated calomel electrode potentiometer copper wire CuSO4 a) Write the half reaction for the Cu electrode. b) Write the Nernst equation for the Cu electrode, which will include [Cu2+] c) If the voltage on the potentiometer reads 0.068 V, solve for [Cu²+].arrow_forward2. (Part B). Identify a sequence of FGI that prepares the Synthesis Target 2,4-dimethoxy- pentane. All carbons in the Synthesis Target must start as carbons in either ethyne, propyne or methanol. Hint: use your analysis of Product carbons' origins (Part A) to identify possible structure(s) of a precursor that can be converted to the Synthesis Target using one FGI. All carbons in the Synthesis Target must start as carbons in one of the three compounds below. H = -H H = -Me ethyne propyne Synthesis Target 2,4-dimethoxypentane MeOH methanol OMe OMe MeO. OMe C₂H₁₂O₂ Product carbons' origins Draw a box around product C's that came from A1. Draw a dashed box around product C's that came from B1.arrow_forwardDraw the skeletal ("line") structure of the smallest organic molecule that produces potassium 3-hydroxypropanoate when reacted with KOH. Click and drag to start drawing a structure. Sarrow_forward
- draw skeletal structures for the minor products of the reaction.arrow_forward1. Provide missing starting materials, reagents, products. If a product cannot be made, write NP (not possible) in the starting material box. C7H12O Ph HO H 1) 03-78 C 2) Me₂S + Ph .H OH + 2nd stereoisomer OH Ph D + enantiomer cat OsO 4 NMO H2O acetonearrow_forwardPlease note that it is correct and explains it rightly:Indicate the correct option. The proportion of O, C and H in the graphite oxide is:a) Constant, for the quantities of functional groups of acids, phenols, epoxy, etc. its constants.b) Depending on the preparation method, as much oxidant as the graphite is destroyed and it has less oxygen.c) Depends on the structure of the graphic being processed, whether it can be more tridimensional or with larger crystals, or with smaller crystals and with more edges.arrow_forward
- Check the box under each a amino acid. If there are no a amino acids at all, check the "none of them" box under the table. Note for advanced students: don't assume every amino acid shown must be found in nature. ནང་་་ OH HO HO NH2 + NH3 O OIL H-C-CO CH3-CH O C=O COOH COOH + H2N C-H O H2N C H CH3-CH CH2 HO H3N O none of them 口 CH3 CH2 OH Хarrow_forwardWhat is the systematic name of the product P of this chemical reaction? 010 HO-CH2-CH2-C-OH ☐ + NaOH P+ H2Oarrow_forward1. Provide missing starting materials, reagents, products. If a product cannot be made, write NP (not possible) in the starting material box. a) C10H12 Ph OMe AcOHg+ + enantiomer Br C6H10O2 + enantiomerarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning


Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole


Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning