
PRINCIPLES OF INSTRUMENTAL ANALYSIS
7th Edition
ISBN: 9789353506193
Author: Skoog
Publisher: CENGAGE L
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 15, Problem 15.15QAP
The following lifetimes were measured for the chloride quenching of quinine sulfate given in Example 15-1.The fluorescence intensities are given in the example.
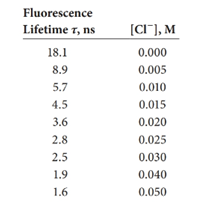
(a) Plot fluorescence intensity versus [Cl-].
(b) Plot the ratio of intensity to lifetime, F-
(c) Develop a normalization factor to correct the measured fluorescence intensity to that of the solution without quencher.
(d) Plot on the same graph Fversus [Cl-] and Fcorr versus (CI-].
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Indicate the coordination forms of Si in silicates.
Briefly indicate the structure and bonding of silicates.
4
Part C
Give the IUPAC name and a common name for the following ether:
Spell out the full names of the compound in the indicated order separated by a comma.
Chapter 15 Solutions
PRINCIPLES OF INSTRUMENTAL ANALYSIS
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Try: Draw possible resonance contributing structures for the following organic species: CH3CH2NO2 [CH2CHCH2] [CH2CHCHO] [CH2CHCH2] [CH2CHNH2]arrow_forwardComplete the following synthesis. (d). H+ ง сarrow_forwardCan the target compound be efficiently synthesized in good yield from the substituted benzene of the starting material? If yes, draw the synthesis. Include all steps and all reactants.arrow_forward
- This is a synthesis question. Why is this method wrong or worse than the "correct" method? You could do it thiss way, couldn't you?arrow_forwardTry: Draw the best Lewis structure showing all non-bonding electrons and all formal charges if any: (CH3)3CCNO NCO- HN3 [CH3OH2]*arrow_forwardWhat are the major products of the following reaction? Draw all the major products. If there are no major products, then there is no reaction that will take place. Use wedge and dash bonds when necessary.arrow_forward
- IX) By writing the appropriate electron configurations and orbital box diagrams briefly EXPLAIN in your own words each one of the following questions: a) The bond length of the Br2 molecule is 2.28 Å, while the bond length of the compound KBr is 3.34 Å. The radius of K✶ is 1.52 Å. Determine the atomic radius in Å of the bromine atom and of the bromide ion. Br = Br b) Explain why there is a large difference in the atomic sizes or radius of the two (Br and Br). Tarrow_forwardWhen 15.00 mL of 3.00 M NaOH was mixed in a calorimeter with 12.80 mL of 3.00 M HCl, both initially at room temperature (22.00 C), the temperature increased to 29.30 C. The resultant salt solution had a mass of 27.80 g and a specific heat capacity of 3.74 J/Kg. What is heat capacity of the calorimeter (in J/C)? Note: The molar enthalpy of neutralization per mole of HCl is -55.84 kJ/mol.arrow_forwardWhen 15.00 mL of 3.00 M NaOH was mixed in a calorimeter with 12.80 mL of 3.00 M HCl, both initially at room temperature (22.00 C), the temperature increased to 29.30 C. The resultant salt solution had a mass of 27.80 g and a specific heat capacity of 3.74 J/Kg. What is heat capacity of the calorimeter (in J/C)? Note: The molar enthalpy of neutralization per mole of HCl is -55.84 kJ/mol. Which experimental number must be initialled by the Lab TA for the first run of Part 1 of the experiment? a) the heat capacity of the calorimeter b) Mass of sample c) Ti d) The molarity of the HCl e) Tfarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Photochemistry : Introduction to Basic Theory of Photochemical Process [Part 1]; Author: Dr. Vikrant Palekar;https://www.youtube.com/watch?v=2NDOL11d6no;License: Standard YouTube License, CC-BY
Photochemistry-1; Author: CH-08:ARYABHATT [Mathematics, Physics, Chemistry];https://www.youtube.com/watch?v=DC4J0t1z3e8;License: Standard Youtube License