
(a)
Interpretation:
The value of the rate constant for the decomposition of sulfuryl chloride at 600 K should be determined.
Concept Introduction:
Integrated rate laws for zero, first and second order reactions are,
Zeroth order:
First order:
Second order:
(a)
Answer to Problem 106AE
Explanation of Solution
If
| Time(hour) | 0.00 | 1.00 | 2.00 | 4.00 | 8.00 | 16.00 |
| Ptotal(atm) | 4.93 | 5.60 | 6.34 | 7.33 | 8.56 | 9.52 |
| PSO2Cl2 | 4.93 | 4.26 | 3.52 | 2.53 | 1.30 | 0.34 |
| Ln PSO2Cl2 | 1.595 | 1.449 | 1.258 | 0.928 | 0.262 | -1.08 |
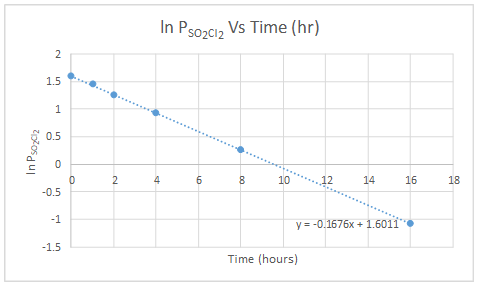
Pressure of gas is directly proportional to concentration.
The graph of ln PSO2Cl2 versus time is linear. So, the reaction of decomposition of SO2Cl2 is first order.
Integrated rate law for the reaction is,
The slope of the graph gives the value for rate constant.
(b)
Interpretation:
The half-life of the reaction should be calculated.
Concept Introduction:
Half-life for first order reaction can be calculated by,
(b)
Answer to Problem 106AE
Explanation of Solution
(c)
Interpretation:
The pressure in the vessel after 0.500 h and after 12.0 h should be calculated.
Concept Introduction:
Integrated rate law for the first order reaction;
[A]t − concentration of A at time t
[A]0 − initial concentration of A
k − rate constant
t − time
(c)
Answer to Problem 106AE
After 0.500 h = 5.33 atm
After 12.00 h = 9.20 atm
Explanation of Solution
After 0.500 h;
After 12.00 h;
(d)
Interpretation:
The fraction of the sulfuryl chloride remains after 20.0 h should be calculated.
Concept Introduction:
Integrated rate law for the first order reaction;
[A]t − concentration of A at time t
[A]0 − initial concentration of A
k − rate constant
t − time
(d)
Answer to Problem 106AE
Fraction of SO2Cl2 left =
Explanation of Solution
Fraction of SO2Cl2 left =
Want to see more full solutions like this?
Chapter 15 Solutions
Chemical Principles
- Can I get helpp drawing my arrowsarrow_forwardWhich of the m/z values corresponds to the base peak in the mass spectrum shown? 100 80 A. 45 B. 44 C. 29 D. 15 Intensity 20 0 10 20 30 40 B- m/z -8 50 E. 30 Which of the m/z values correspond to the molecular ion for the compound shown? A. 18 B. 82 OH C. 100 D. 102 E. 103arrow_forwardCan someone help me with drawing my arrows.arrow_forward
- I'm having trouble with converting lewis diagrams into VSEPR diagrams. I currently have this example of C2BrCl3 which I want to turn into a lewis structure, but I'm not sure what steps I need to do in order to do so. I have the table written down, however, there's two central atoms so what would I do? There seems to be 4 electron domains on the carbon atom and no lone pairs so it would seem like this shape would be tetrahedral. Here's what I have now. Thanks!arrow_forwardWe discussed the solid phase resin using in peptide synthesis. Provide a mechanism, for its formation. DRAW THE MECHANISM.arrow_forwardPlease help. Every time I've asked an expert in the past, it's been wrong :(arrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





