
Organic Chemistry (6th Edition)
6th Edition
ISBN: 9781260119107
Author: Janice Gorzynski Smith
Publisher: McGraw Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 14.12, Problem 20P
Draw the product formed when each diene and dienophile react in a Diels–Alder reaction.
a. 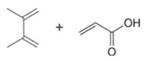 b.
b. 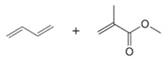 c.
c. 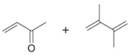
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
By malonic or acetylacetic synthesis, synthesize 5-Methyl-2-hexanone (with the formulas of the compounds).
QUESTION: Answer Question 5: 'Calculating standard error of regression' by filling in all the empty green boxes
*The values are all provided in the first photo attached*
Draw the formula for 3-chlorobenzoic acetic anhydride.
Chapter 14 Solutions
Organic Chemistry (6th Edition)
Ch. 14.1 - Prob. 2PCh. 14.2 - Problem 16.3 Draw a second resonance structure for...Ch. 14.2 - Prob. 4PCh. 14.2 - Problem 16.5 Farnesyl diphosphate is synthesized...Ch. 14.3 - Prob. 6PCh. 14.4 - Prob. 7PCh. 14.4 - Prob. 8PCh. 14.8 - Problem 16.12 Using hybridization, predict how the...Ch. 14.8 - Problem 16.13 Use resonance theory to explain why...Ch. 14.9 - Prob. 15P
Ch. 14.9 - Prob. 16PCh. 14.10 - Problem 16.17 Draw a stepwise mechanism for the...Ch. 14.11 - Prob. 19PCh. 14.12 - Problem 16.19 Draw the product formed when each...Ch. 14 - Prob. 32PCh. 14 - 16.31 Which of the following systems are...Ch. 14 - 16.32 Draw all reasonable resonance structures for...Ch. 14 - Prob. 35PCh. 14 - 16.35 Explain why the cyclopentadienide anion A...Ch. 14 - Prob. 38PCh. 14 - 16.37 Draw the structure of each compound.
a. in...Ch. 14 - 16.41 Draw the products formed when each compound...Ch. 14 - Prob. 44PCh. 14 - 16.43 Treatment of alkenes A and B with gives the...Ch. 14 - 16.44 Draw a stepwise mechanism for the following...Ch. 14 - Prob. 47PCh. 14 - 16.57 A transannular Diels–Alder reaction is an...Ch. 14 - Draw a stepwise mechanism for the following...Ch. 14 - Prob. 62PCh. 14 - Prob. 63PCh. 14 - Prob. 64PCh. 14 - Prob. 65PCh. 14 - 16.65 The treatment of isoprene with one...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- By malonic or acetylacetic synthesis, synthesize 2-methylbutanoic acid (indicate the formulas of the compounds).arrow_forwardObtain 2-methylbutanoic acid by malonic or acetylacetic synthesis (indicate the formulas of the compounds involved).arrow_forwardEFFICIENTS SAMPLE READINGS CONCENTRATIONS Pigiadient) TOMATO SAUCE (REGULAR) TOMATO (REDUCED SALT) TOMATO SAUCE (REGULAR) TOMATO (REDUCED SALT) 58 6.274 3.898 301.7 151.2 14150 5.277 3.865 348.9 254.8 B 5.136 3.639 193.7 85.9 605 4.655 3.041 308.6 199.6 05 5.135 3.664 339.5 241.4 0139 4.676 3.662 160.6 87.6 90148 5.086 3.677 337.7 242.5 0092 6.348 3.775 464.7 186.4 PART3 5.081 3.908 223.5 155.8 5.558 3.861 370.5 257.1 4.922 3.66 326.6 242.9 4.752 3.641 327.5 253.3 50 5.018 3.815 336.1 256.0 84 4.959 3.605 317.9 216.6 38 4.96 3.652 203.8 108.7 $3 5.052 3.664 329.8 239.0 17 5.043 3.767 221.9 149.7 052 5.058 3.614 331.7 236.4 5.051 4.005 211.7 152.1 62 5.047 3.637 309.6 222.7 5.298 3.977 223.4 148.7 5.38 4.24 353.7 278.2 5 5.033 4.044 334.6 268.7 995 4.706 3.621 305.6 234.4 04 4.816 3.728 340.0 262.7 16 4.828 4.496 304.3 283.2 0.011 4.993 3.865 244.7 143.6 AVERAGE STDEV COUNT 95% CI Confidence Interval (mmol/L) [Na+] (mg/100 mL) 95% Na+ Confidence Interval (mg/100 mL)arrow_forward
- If we have two compounds: acetone (CH₃COCH₃) and acetic acid (CH₃COOH), applying heat to them produces an aldol condensation of the two compounds. If this is correct, draw the formula for the final product.arrow_forwardIf we have two compounds: acetone (CH3COCH3) and acetic acid (CH3COOH); if we apply heat (A), what product(s) are obtained?arrow_forwardQUESTION: Fill out the answers to the empty green boxes attached in the image. *Ensure you all incorporate all 27 values (per column)*arrow_forward
- You need to make a buffer by dissolving benzoic acid and sodium benzoate in water. What is the mass of benzoic acid that you would weigh out, in mg, to create 50 mL of a buffer at pH = 4.7 that will change pH no more than 0.10 units with the addition of 0.001 moles of acid or base? Enter just the answer without the units (mg) - just the number will do!arrow_forwardDraw the formula for 3-isopropylcyclopentane-1-carbonyl chloride.arrow_forwardQUESTION: Fill out the answers to the empty green boxes attached in the image. *Ensure you all incorporate all 27 values (per column)*arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY