
Organic Chemistry (6th Edition)
6th Edition
ISBN: 9781260119107
Author: Janice Gorzynski Smith
Publisher: McGraw Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 14.1, Problem 2P
Interpretation Introduction
Interpretation: The given species is to be identified as conjugated.
Concept Introduction: Diene is a hydrocarbon that contains two
Conjugated diene consists of two double bonds that are separated by a single bond, whereas isolated diene also consists of two double bond but they are separated by two more carbon atoms.
The example that shows the basic difference between conjugated diene and isolated diene is shown below.
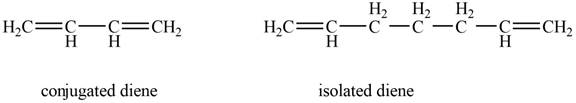
Figure 1
Allylic carbocation is also an example of conjugated system.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Recognizing ampli
Draw an a amino acid with a methyl (-CH3) side chain.
Explanation
Check
Click and drag to start drawing a
structure.
X
C
Write the systematic name of each organic molecule:
structure
name
×
HO
OH
☐
OH
CI
CI
O
CI
OH
OH
く
Check the box under each a amino acid.
If there are no a amino acids at all, check the "none of them" box under the table.
Note for advanced students: don't assume every amino acid shown must be found in nature.
COO
H3N-C-H
CH2
HO
CH3
NH3 O
CH3-CH
CH2
OH
Onone of them
Explanation
Check
+
H3N
O
0.
O
OH
+
NH3
CH2
CH3-CH
H2N C-COOH
H
O
HIC
+
C=O
H3N-C-O
CH3- - CH
CH2
OH
Х
2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Acces
Chapter 14 Solutions
Organic Chemistry (6th Edition)
Ch. 14.1 - Prob. 2PCh. 14.2 - Problem 16.3 Draw a second resonance structure for...Ch. 14.2 - Prob. 4PCh. 14.2 - Problem 16.5 Farnesyl diphosphate is synthesized...Ch. 14.3 - Prob. 6PCh. 14.4 - Prob. 7PCh. 14.4 - Prob. 8PCh. 14.8 - Problem 16.12 Using hybridization, predict how the...Ch. 14.8 - Problem 16.13 Use resonance theory to explain why...Ch. 14.9 - Prob. 15P
Ch. 14.9 - Prob. 16PCh. 14.10 - Problem 16.17 Draw a stepwise mechanism for the...Ch. 14.11 - Prob. 19PCh. 14.12 - Problem 16.19 Draw the product formed when each...Ch. 14 - Prob. 32PCh. 14 - 16.31 Which of the following systems are...Ch. 14 - 16.32 Draw all reasonable resonance structures for...Ch. 14 - Prob. 35PCh. 14 - 16.35 Explain why the cyclopentadienide anion A...Ch. 14 - Prob. 38PCh. 14 - 16.37 Draw the structure of each compound.
a. in...Ch. 14 - 16.41 Draw the products formed when each compound...Ch. 14 - Prob. 44PCh. 14 - 16.43 Treatment of alkenes A and B with gives the...Ch. 14 - 16.44 Draw a stepwise mechanism for the following...Ch. 14 - Prob. 47PCh. 14 - 16.57 A transannular Diels–Alder reaction is an...Ch. 14 - Draw a stepwise mechanism for the following...Ch. 14 - Prob. 62PCh. 14 - Prob. 63PCh. 14 - Prob. 64PCh. 14 - Prob. 65PCh. 14 - 16.65 The treatment of isoprene with one...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Write the systematic name of each organic molecule: structure HO-C-CH2-CH3 O -OH CH3-CH2-CH2-CH2-CH2-C-OH CH3 CH3-CH-CH2-C-OH Explanation Check S namearrow_forwardtheres 2 productsarrow_forwardDraw the major product of this solvolysis reaction. Ignore any inorganic byproducts. + CH3CH2OH Drawing Q Atoms, Bonds and Rings OCH2CH3 || OEt Charges OH 00-> | Undo Reset | Br Remove Done Drag To Pan +arrow_forward
- Draw the major product of this SN1 reaction. Ignore any inorganic byproducts. CH3CO2Na CH3CO2H Drawing + Br Q Atoms, Bonds and Rings OAC Charges OH ОАс Na ဂ Br Undo Reset Remove Done Drag To Pan +arrow_forwardOrganic Functional Groups entifying positions labeled with Greek letters in acids and derivatives 1/5 ssible, replace an H atom on the a carbon of the molecule in the drawing area with a ce an H atom on the ẞ carbon with a hydroxyl group substituent. ne of the substituents can't be added for any reason, just don't add it. If neither substi er the drawing area. O H OH Oneither substituent can be added. Check D 1 Accessibility ado na witharrow_forwardDifferentiate between electrophilic and nucleophilic groups. Give examples.arrow_forward
- An aldehyde/ketone plus an alcohol gives a hemiacetal, and an excess of alcohol gives an acetal. The reaction is an equilibrium; in aldehydes, it's shifted to the right and in ketones, to the left. Explain.arrow_forwardDraw a Haworth projection or a common cyclic form of this monosaccharide: H- -OH H- OH H- -OH CH₂OHarrow_forwardAnswer the question in the first photoarrow_forward
- Ggggffg2258555426855 please don't use AI Calculate the positions at which the probability of a particle in a one-dimensional box is maximum if the particle is in the fifth energy level and in the eighth energy level.arrow_forwardExplain the concepts of hemiacetal and acetal.arrow_forwardBriefly describe a nucleophilic addition.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning