
Organic Chemistry (6th Edition)
6th Edition
ISBN: 9781260119107
Author: Janice Gorzynski Smith
Publisher: McGraw Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 14, Problem 58P
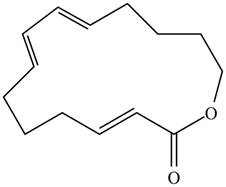
A transannular Diels–Alder reaction is an intramolecular reaction that occurs when the diene and dienophile are contained in one ring, resulting in the formation of a tricyclic ring system. Draw the product formed when the following triene undergoes a transannular Diels–Alder reaction.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
3. Is 2-methyl-2-propanol a primary, secondary, or tertiary alcohol? Write
out the
structures of 2-methyl-2-propanol and also any oxidation products of 2-
methyl-2-
propanol. If there is more than one oxidation product, give the structure of
each
of the products.
4. 2-Propanol is the IUPAC systematic name of this alcohol. It has a
common name
by which it is much better known (You'll see it in the grocery store or
pharmacy).
Give that common name
5. Aldehydes can be synthesized by the oxidation of. Please choose from
below choices
A. Primary alcohols
B. Secondary alcohols
C. Organic acids
D. Inorganic acids
6. Tertiary alcohol Can undergo oxidation. yes or no. ? If yes then answer the
product.
Finish the reactions
hand written please
Part A
Identify each alcohol as primary, secondary, or tertiary
Drag the appropriate items to their respective bins.
CH₂
H₂C-
-C-OH
HO
CH₂
Primary
Он
OH
CH₂
OH
CCH₂OH
CH₂
сн
Secondary
Tertiary
Reset Help
CH,CH₂
(CH)CHCH,OH CH,CH,CH,CCH,
CHOH
CH₂
Different types of alcohol groups
Alcohol and its reaction:
8. Combing two alcohol molecules below and completing the reaction with
Product .( Hint Reaction called etherification as ether is formed and name the
ether once you complete the reaction.
Hint.:
R-O-H+H-O-RR-O-R
Do the reaction:
CH₂OH + CH₂OH---→
+ H-O-H
9. Write the reaction of formation of alcohol from alkene by adding water:
Addition reaction also called hydration reaction as we are adding water
which occur always in presence of acid
Hint: Break the double bond and add H and OH if symmetrical then
add anywhere if unsymmetrical then follow Markovnikov rule H
should go to that double bone carbon which has more hydrogen
CH2=CH2 + H₂O-→
Chapter 14 Solutions
Organic Chemistry (6th Edition)
Ch. 14.1 - Prob. 2PCh. 14.2 - Problem 16.3 Draw a second resonance structure for...Ch. 14.2 - Prob. 4PCh. 14.2 - Problem 16.5 Farnesyl diphosphate is synthesized...Ch. 14.3 - Prob. 6PCh. 14.4 - Prob. 7PCh. 14.4 - Prob. 8PCh. 14.8 - Problem 16.12 Using hybridization, predict how the...Ch. 14.8 - Problem 16.13 Use resonance theory to explain why...Ch. 14.9 - Prob. 15P
Ch. 14.9 - Prob. 16PCh. 14.10 - Problem 16.17 Draw a stepwise mechanism for the...Ch. 14.11 - Prob. 19PCh. 14.12 - Problem 16.19 Draw the product formed when each...Ch. 14 - Prob. 32PCh. 14 - 16.31 Which of the following systems are...Ch. 14 - 16.32 Draw all reasonable resonance structures for...Ch. 14 - Prob. 35PCh. 14 - 16.35 Explain why the cyclopentadienide anion A...Ch. 14 - Prob. 38PCh. 14 - 16.37 Draw the structure of each compound.
a. in...Ch. 14 - 16.41 Draw the products formed when each compound...Ch. 14 - Prob. 44PCh. 14 - 16.43 Treatment of alkenes A and B with gives the...Ch. 14 - 16.44 Draw a stepwise mechanism for the following...Ch. 14 - Prob. 47PCh. 14 - 16.57 A transannular Diels–Alder reaction is an...Ch. 14 - Draw a stepwise mechanism for the following...Ch. 14 - Prob. 62PCh. 14 - Prob. 63PCh. 14 - Prob. 64PCh. 14 - Prob. 65PCh. 14 - 16.65 The treatment of isoprene with one...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Complete the reaction hand written pleasearrow_forwardPredict the major products of this organic reaction: HBr (1 equiv) cold ? Some important notes: • Draw the major product, or products, of this reaction in the drawing area below. • You can draw the products in any arrangement you like. • Pay careful attention to the reaction conditions, and only include the major products. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. • Note that there is only 1 equivalent of HBr reactant, so you need not consider the case of multiple additions. dm Re Explanation Check ©2025 McGraw Hill LLC. All Rights Reserved. Termarrow_forwardb) Use curved arrows to show the reaction of the radical with hydrogen bromide. Br: Br H .. Answer Bankarrow_forward
- Indicate the products of the reaction between CH3COCH2COONa (Sodium acetoacetate) and BrCH2COOC2H5arrow_forwardIndicate whether the product of the reaction between Naphthalene and CrO3 in acetic acid at 25ºC is 1,4 naphthoquinone or phthalic anhydride.arrow_forwardIndicate the products of the reaction between CH3COCH2COOC2H5 and Na+-OC2H5.arrow_forward
- Primary, Secondary, and Tertiary Alcohols O-H O-H O-H R₁-C-H R₁-C-H R₁-C-R₁ H R₂ R₂ Primary Alcohol Secondary Alcohol ChemistryLearner.com R stands for Carbon group like ethyl methyl propyl Tertiary Alcohol If 1 carbon group with two H attached to alcoholic carbon, then primary If 2 carbon group and 1 H are attached to alcoholic carbon, then secondary IF 3 carbon group and no H attach to alcoholic carbon then tertiary. The bottom line Starting "Weak" oxidant material PCC, DMP, Swern, etc Primary alcohol Aldehyde OH Secondary alcohol Ketone OH "Strong" oxidant KMnO4, H₂CrO4 (or equivalent) OH Carboxylic acid 요 Ketone No reaction No reaction Tertiary alcohol 1. Is ethanol a primary, secondary, or tertiary alcohol? Write out the structures of ethanol and any oxidation products of ethanol. If there is more than one oxidation product, give the structure of each of the products. 2. Is 2-propanol a primary, secondary, or tertiary alcohol? Write out the structures of 2-propanol and any…arrow_forwardFormulate the reaction: Naphthalene with CrO3 in acetic acid at 25ºCarrow_forwardComplete the reaction hand written pleasearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Alcohols, Ethers, and Epoxides: Crash Course Organic Chemistry #24; Author: Crash Course;https://www.youtube.com/watch?v=j04zMFwDeDU;License: Standard YouTube License, CC-BY