
Concept explainers
(a)
Interpretation:
The oxidized product of the following alcohol when oxidized with
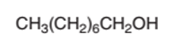
Concept Introduction:
A
In a chemical reaction, the substance which is involved in conversion is said to be reactant whereas the newly formed substance is known as a product. Both reactants and products must be separated by an arrow.
The oxidation reaction is the reaction that involves the addition of O atom in the presence of certain oxidizing agents such as
Answer to Problem 63P
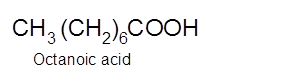
Explanation of Solution
To get the oxidized product of any alcohol, three steps must be followed;
- Locate the C atom in the parent chain that is bonded with −OH group.
- Convert that C atom to carbonyl C atom or
carboxylic acid as it is overall removal of H atoms. - Primary alcohols are oxidized to
aldehyde which further oxidized to a carboxylic acid. - Secondary alcohols are oxidized to a
ketone (R2CO). - Tertiary alcohols are not oxidized as they do not have H atom on the C with the −OH group.
Hence, the oxidization of 1-octanol will form octanoic acid molecule as 1-octanol is a primary alcohol.
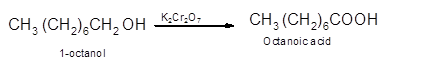
(b)
Interpretation:
The oxidized product of the following alcohol when oxidized with
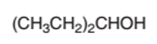
Concept Introduction:
A chemical reaction is the symbolic representation of the conversion of substances to new substances.
In a chemical reaction, the substance which is involved in conversion is said to be reactant whereas the newly formed substance is known as a product. Both reactants and products must be separated by an arrow.
The oxidation reaction is the reaction that involves the addition of O atom in the presence of certain oxidizing agents such as
Answer to Problem 63P
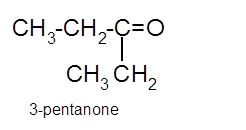
Explanation of Solution
To get the oxidized product of any alcohol, three steps must be followed;
- Locate the C atom in the parent chain that is bonded with −OH group.
- Convert that C atom to carbonyl C atom or carboxylic acid as it is overall removal of H atoms.
- Primary alcohols are oxidized to aldehyde which further oxidized to a carboxylic acid.
- Secondary alcohols are oxidized to a ketone (R2CO).
- Tertiary alcohols are not oxidized as they do not have H atom on the C with the −OH group.
Hence the oxidization of 3-pentanol will form 3-pentanone molecule as 3-pentanol is a secondary alcohol.
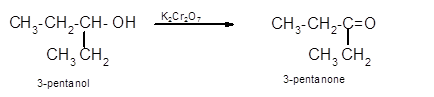
(c)
Interpretation:
The oxidized product of the following alcohol when oxidized with
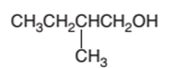
Concept Introduction:
A chemical reaction is the symbolic representation of the conversion of substances to new substances.
In a chemical reaction, the substance which is involved in conversion is said to be reactant whereas the newly formed substance is known as a product. Both reactants and products must be separated by an arrow.
The oxidation reaction is the reaction that involves the addition of O atom in the presence of certain oxidizing agents such as
Answer to Problem 63P
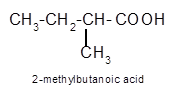
Explanation of Solution
To get the oxidized product of any alcohol, three steps must be followed;
- Locate the C atom in the parent chain that is bonded with −OH group.
- Convert that C atom to carbonyl C atom or carboxylic acid as it is overall removal of H atoms.
- Primary alcohols are oxidized to aldehyde which further oxidized to the carboxylic acid.
- Secondary alcohols are oxidized to ketone (R2CO).
- Tertiary alcohols are not oxidized as they do not have H atom on the C with the −OH group.
Hence, the oxidization of 3-pentanol will form 3-pentanone molecule as 3-pentanol is a secondary alcohol.
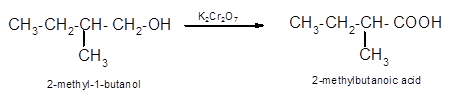
(d)
Interpretation:
The oxidized product of following alcohol when oxidized with
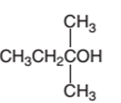
Concept Introduction:
A chemical reaction is the symbolic representation of the conversion of substances to new substances.
In a chemical reaction, the substance which is involved in conversion is said to be reactant whereas the newly formed substance is known as a product. Both reactants and products must be separated by an arrow.
Oxidation reaction is the reaction that involves the addition of O atom in the presence of certain oxidizing agents such as
Answer to Problem 63P
2-ethyl-2-propanol cannot oxidize as it is a tertiary alcohol.
Explanation of Solution
To get the oxidized product of any alcohol, three steps must be followed;
- Locate the C atom in the parent chain that is bonded with −OH group.
- Convert that C atom to carbonyl C atom or carboxylic acid as it is the overall removal of H atoms.
- Primary alcohols are oxidized to aldehyde which further oxidized to the carboxylic acid.
- Secondary alcohol is oxidized to ketone (R2CO).
- Tertiary alcohols are not oxidized as they do not have H atom on the C with the −OH group.
Hence, 2-ethyl-2-propanol cannot oxidize as it is a tertiary alcohol.
Want to see more full solutions like this?
Chapter 14 Solutions
CONNECT IA GENERAL ORGANIC&BIO CHEMISTRY
- Predict the major products of this organic reaction: HBr (1 equiv) cold ? Some important notes: • Draw the major product, or products, of this reaction in the drawing area below. • You can draw the products in any arrangement you like. • Pay careful attention to the reaction conditions, and only include the major products. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. • Note that there is only 1 equivalent of HBr reactant, so you need not consider the case of multiple additions. dm Re Explanation Check ©2025 McGraw Hill LLC. All Rights Reserved. Termarrow_forwardb) Use curved arrows to show the reaction of the radical with hydrogen bromide. Br: Br H .. Answer Bankarrow_forwardIndicate the reaction products when CH3COCH2COOCH2COOC2H5 (ethyl acetoacetoacetate) reacts with 1º OH-/H2O and 2º H3O+arrow_forward
- Indicate whether the product of the reaction between Naphthalene and CrO3 in acetic acid at 25ºC is 1,4 naphthoquinone or phthalic anhydride.arrow_forwardIndicate the products of the reaction between CH3COCH2COOC2H5 and Na+-OC2H5.arrow_forwardPrimary, Secondary, and Tertiary Alcohols O-H O-H O-H R₁-C-H R₁-C-H R₁-C-R₁ H R₂ R₂ Primary Alcohol Secondary Alcohol ChemistryLearner.com R stands for Carbon group like ethyl methyl propyl Tertiary Alcohol If 1 carbon group with two H attached to alcoholic carbon, then primary If 2 carbon group and 1 H are attached to alcoholic carbon, then secondary IF 3 carbon group and no H attach to alcoholic carbon then tertiary. The bottom line Starting "Weak" oxidant material PCC, DMP, Swern, etc Primary alcohol Aldehyde OH Secondary alcohol Ketone OH "Strong" oxidant KMnO4, H₂CrO4 (or equivalent) OH Carboxylic acid 요 Ketone No reaction No reaction Tertiary alcohol 1. Is ethanol a primary, secondary, or tertiary alcohol? Write out the structures of ethanol and any oxidation products of ethanol. If there is more than one oxidation product, give the structure of each of the products. 2. Is 2-propanol a primary, secondary, or tertiary alcohol? Write out the structures of 2-propanol and any…arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning





