
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN: 9781938168390
Author: Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher: OpenStax
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 14, Problem 44E
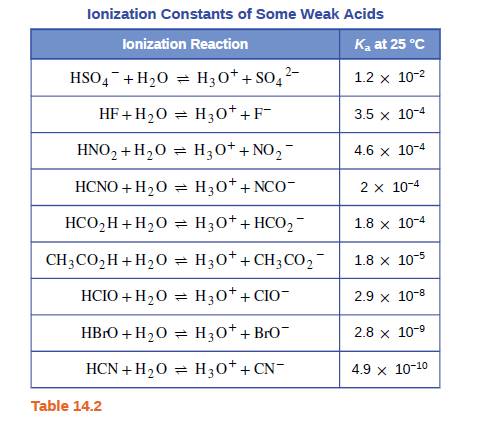
Both HF and HCN ionize in water to a limited extent. Which of the conjugate bases. F“ or CN”, is the stronger base? See Table 14.2.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
pressure (atm)
3
The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the
temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes.
0
0
200
temperature (K)
400
а
er your payment details | bar xb Home | bartleby
x +
aleksogi/x/isl.exe/1o u-lgNskr7j8P3jH-1Qs_pBanHhviTCeeBZbufuBYT0Hz7m7D3ZcW81NC1d8Kzb4srFik1OUFhKMUXzhGpw7k1
O States of Matter
Sketching a described thermodynamic change on a phase diagram
0/5
The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the
temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes.
pressure (atm)
1
3-
0-
0
200
Explanation
Check
temperature (K)
400
X
Q Search
L
G
2025 McGraw Hill LLC. All Rights Reserved Terms of Use Privacy Ce
5.
Chapter 14 Solutions
Chemistry by OpenStax (2015-05-04)
Ch. 14 - Write equations that show NH3 as both a conjugate...Ch. 14 - Write equations that show H2PO4- acting both as an...Ch. 14 - Show by suitable net ionic equations that each of...Ch. 14 - Show by suitable net ionic equations that each of...Ch. 14 - Show by suitable net ionic equations that each of...Ch. 14 - Show by suitable net ionic equations that each of...Ch. 14 - What is the conjugate acid of each of the...Ch. 14 - What is the conjugate acid of each of the...Ch. 14 - Identify and label the Bronsted-Lowry acid, its...Ch. 14 - Identify and label the Bronsted-Lowry acid, its...
Ch. 14 - What are amphiprotic species? Illustrate with...Ch. 14 - State which of the following species are...Ch. 14 - State which of the following species are...Ch. 14 - Is the self-ionization of water endothermic or...Ch. 14 - Explain why a sample of pure water at 40 C is...Ch. 14 - The ionization constant for water (Kw) is 2.91014...Ch. 14 - The ionization constant for water (Kw) is...Ch. 14 - Calculate the pH and the pOH of each of the...Ch. 14 - Calculate the pH and the pOH of each of the...Ch. 14 - What are the pH and pOH of a solution of 2.0 M...Ch. 14 - What are the hydronium and hydroxide ion...Ch. 14 - Calculate the hydrogen ion concentration and the...Ch. 14 - Calculate the hydronium ion concentration and the...Ch. 14 - The hydronium ion concentration in a sample of...Ch. 14 - The hydroxide ion concentration in household...Ch. 14 - Explain why the neutralization reaction of a...Ch. 14 - Explain why the neutralization reaction of a weak...Ch. 14 - Use this list of important industrial compounds...Ch. 14 - The odor of vinegar is due to the presence of...Ch. 14 - Household ammonia is a solution of the weak base...Ch. 14 - Explain why the ionization constant, Ka, for H2SO4...Ch. 14 - Explain why the ionization constant, Ka, for HI is...Ch. 14 - Gastric juice, the digestive ?uid produced in the...Ch. 14 - Nitric acid reacts with insoluble copper (II)...Ch. 14 - What is the ionization constant at 25 C for the...Ch. 14 - What is the ionization constant at 25 C for the...Ch. 14 - Which base, CH3NH2 or (CH3)2NH, is the stronger...Ch. 14 - Which is the stronger acid, NH4+ or HBrO?Ch. 14 - Which is the stronger base, (CH3)3N or H2BO3-?Ch. 14 - Predict which acid in each of the following pairs...Ch. 14 - Predict which compound in each of the following...Ch. 14 - Rank the compounds in each of the following groups...Ch. 14 - Rank the compounds in each of the following groups...Ch. 14 - Both HF and HCN ionize in water to a limited...Ch. 14 - The active ingredient formed by aspirin in the...Ch. 14 - What do we represent when we write:...Ch. 14 - Explain why equilibrium calculations are not...Ch. 14 - Are the concentrations of hydronium ion and...Ch. 14 - What two common assumptions can simplify...Ch. 14 - What two common assumptions can simplify...Ch. 14 - Which of the following will increase the percent...Ch. 14 - Which of the following will increase the percent...Ch. 14 - What is the effect on the concentrations of NO2-,...Ch. 14 - What is the effect on the concentration of...Ch. 14 - Why is the hydronium ion concentration in a...Ch. 14 - From the equilibrium concentrations given,...Ch. 14 - From the equilibrium concentrations given,...Ch. 14 - Determine Kb for the nitrite ion, NO2-. In a...Ch. 14 - Determine Ka for hydrogen sulfate ion, HSO4-. In a...Ch. 14 - Calculate the ionization constant for each of the...Ch. 14 - Calculate the ionization constant for each of the...Ch. 14 - For which of the following solutions must we...Ch. 14 - Even though both NH3 and C6H5NH2 are weak bases,...Ch. 14 - Calculate the equilibrium concentration of the...Ch. 14 - Calculate the equilibrium concentration of the...Ch. 14 - Calculate the equilibrium concentration of the...Ch. 14 - Calculate the equilibrium concentration of the...Ch. 14 - Using the Ka value of , place Al(H2O)63+ in the...Ch. 14 - Calculate the concentration of all solute species...Ch. 14 - Propionic acid, C2H5CO2H (Ka=1.34105), is used in...Ch. 14 - White vinegar is a 5.0% by mass solution of acetic...Ch. 14 - The ionization constant of lactic acid,...Ch. 14 - Nicotine, C10H14N2, is a base that will accept two...Ch. 14 - The pH of a 0.20-M solution of HP is 1.92....Ch. 14 - The pH of a 0.15-M solution of HSO4- is 1.43....Ch. 14 - The pH of a 0.10-M solution of caffeine is 11.16....Ch. 14 - Tile pH of a solution of household ammonia, a...Ch. 14 - Determine whether aqueous solutions of the...Ch. 14 - Determine whether aqueous solutions of the...Ch. 14 - Novocaine, C13H21O2N2Cl, is the salt of the base...Ch. 14 - Which of the following concentrations would be...Ch. 14 - Calculate the concentration of each species...Ch. 14 - Calculate the concentration of each species...Ch. 14 - Salicylic acid, HOC6H4CO2H, and its derivatives...Ch. 14 - The ion HTe- is an amphiprotic species; it can act...Ch. 14 - Explain why a buffer can be prepared from a...Ch. 14 - Explain why the pH does not change significantly...Ch. 14 - Explain why the pH does not change significantly...Ch. 14 - What is [H3O+] in a solution of 0.25 M CH3CO2H and...Ch. 14 - What is [H3O+] in a solution of 0.075 M HNO2 and...Ch. 14 - What is [OH-] in a solution of 0.125 M CH3NH2 and...Ch. 14 - What is [OH-] in a solution of 1.25 M NH3 and 0.78...Ch. 14 - What concentration of NH4NO3 is required to make...Ch. 14 - What concentration of NaF is required to make...Ch. 14 - What is the effect on the concentration of acetic...Ch. 14 - What is the effect on the concentration of...Ch. 14 - What will be the pH of a buffer solution prepared...Ch. 14 - Calculate the pH of a buffer solution prepared...Ch. 14 - How much solid NaCH3CO23H2O must be added to 0300...Ch. 14 - What mass of NH4Cl must be added to 0.750 L of a...Ch. 14 - A buffer solution is prepared from equal volumes...Ch. 14 - A 5.36-g sample of NH4Cl was added to 25.0 mL of...Ch. 14 - Which acid in Table 14.2 is most appropriate for...Ch. 14 - Which acid in Table 14.2 is most appropriate for...Ch. 14 - Which base in Table 14.3 is must appropriate for...Ch. 14 - Which base in Table 14.3 is most appropriate for...Ch. 14 - Saccharin, C7H4NSO3H, is a weak acid (Ka=2.1102)....Ch. 14 - What is the pH of 1.000 L of a solution of 100.0 g...Ch. 14 - Explain how to choose the appropriate acid-base...Ch. 14 - Explain why an acid-base indicator changes color...Ch. 14 - Why can we ignore the contribution of water to the...Ch. 14 - Why can we ignore the contribution of water to the...Ch. 14 - Draw a curve for a series of solutions of HF. Plot...Ch. 14 - Draw a curve similar to that shown in Figure 14.23...Ch. 14 - Calculate the pH at the following points in a...Ch. 14 - The indicator dinitrophenol is an acid with a Ka...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Endospore formation is called (a) _____. It is initiated by (b) _____. Formation of a new cell from an endospor...
Microbiology: An Introduction
Q1. Which wavelength of light has the highest frequency?
a) 10 nm
b) 10 mm
c) 1 nm
d) 1 mm
Chemistry: A Molecular Approach (4th Edition)
Level 2: Application/Analysis 4. Nitrifying bactcria participatc in the nitrogen cycle mainly by (A) converting...
Campbell Biology (11th Edition)
For each reaction, calculate how many moles of product from when 1.75 mol of the reactant in color completely r...
Introductory Chemistry (6th Edition)
How can the freezing of water crack boulders?
Campbell Biology in Focus (2nd Edition)
What type of cut would separate the brain into anterior and posterior parts?
Anatomy & Physiology (6th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 6.arrow_forward0/5 alekscgi/x/sl.exe/1o_u-IgNglkr7j8P3jH-IQs_pBaHhvlTCeeBZbufuBYTi0Hz7m7D3ZcSLEFovsXaorzoFtUs | AbtAURtkqzol 1HRAS286, O States of Matter Sketching a described thermodynamic change on a phase diagram The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 3 pressure (atm) + 0- 0 5+ 200 temperature (K) 400 Explanation Check X 0+ F3 F4 F5 F6 F7 S 2025 McGraw Hill LLC All Rights Reserved. Terms of Use Privacy Center Accessibility Q Search LUCR + F8 F9 F10 F11 F12 * % & ( 5 6 7 8 9 Y'S Dele Insert PrtSc + Backsarrow_forward5.arrow_forward
- 9arrow_forwardalekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-IQs_pBanHhvlTCeeBZbufu BYTI0Hz7m7D3ZS18w-nDB10538ZsAtmorZoFusYj2Xu9b78gZo- O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 3- 200 temperature (K) Explanation Chick Q Sowncharrow_forward0+ aleksog/x/lsl.exe/1ou-lgNgkr7j8P3H-IQs pBaHhviTCeeBZbufuBYTOHz7m7D3ZStEPTBSB3u9bsp3Da pl19qomOXLhvWbH9wmXW5zm O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 Gab The temperature on a sample of pure X held at 0.75 atm and -229. °C is increased until the sample sublimes. The temperature is then held constant and the pressure is decreased by 0.50 atm. On the phase diagram below draw a path that shows this set of changes. F3 pressure (atm) 0- 0 200 Explanation temperature (K) Check F4 F5 ☀+ Q Search Chill Will an 9 ENG F6 F7 F8 F9 8 Delete F10 F11 F12 Insert PrtSc 114 d Ararrow_forward
- x + LEKS: Using a phase diagram a X n/alekscgi/x/lsl.exe/10_u-IgNsikr7j8P3jH-IQs_pBan HhvlTCeeBZbufu BYTI0Hz7m7D3ZcHYUt80XL-5alyVpw ○ States of Matter Using a phase diagram to find a phase transition temperature or pressure Use the phase diagram of Substance X below to find the melting point of X when the pressure above the solid is 1.1 atm. pressure (atm) 16 08- solid liquid- 0 200 400 gas 600 temperature (K) Note: your answer must be within 25 °C of the exact answer to be graded correct. × 5arrow_forwardS: Using a phase diagram leksogi/x/sl.exe/1ou-IgNs kr 7j8P3jH-IQs_pBan HhvTCeeBZbufuBYTI0Hz7m7D3ZdHYU+80XL-5alyVp O States of Matter Using a phase diagram to find a phase transition temperature or pressure se the phase diagram of Substance X below to find the boiling point of X when the pressure on the liquid is 1.6 atm. pressure (atm) 32- 16- solid liquid 0. gas 100 200 temperature (K) 300 Note: your answer must be within 12.5 °C of the exact answer to be graded correct. 10 Explanation Check § Q Search J 2025 McGraw Hill LLC. All Rights Researrow_forward151.2 254.8 85.9 199.6 241.4 87.6 242.5 186.4 155.8 257.1 242.9 253.3 256.0 216.6 108.7 239.0 149.7 236.4 152.1 222.7 148.7 278.2 268.7 234.4 262.7 283.2 143.6 QUESTION: Using this group of data on salt reduced tomato sauce concentration readings answer the following questions: 1. 95% Cl Confidence Interval (mmol/L) 2. [Na+] (mg/100 mL) 3. 95% Na+ Confidence Interval (mg/100 mL)arrow_forward
- Results Search Results Best Free Coursehero Unloc xb Success Confirmation of Q x O Google Pas alekscgi/x/lsl.exe/1o_u-IgNslkr 7j8P3jH-IQs_pBanHhvlTCeeBZbufu BYTI0Hz7m7D3ZcHYUt80XL-5alyVpwDXM TEZayFYCavJ17dZtpxbFD0Qggd1J O States of Matter Using a phase diagram to find a phase transition temperature or pressure Gabr 3/5 he pressure above a pure sample of solid Substance X at 101. °C is lowered. At what pressure will the sample sublime? Use the phase diagram of X below to nd your answer. pressure (atm) 24- 12 solid liquid gas 200 400 temperature (K) 600 ote: your answer must be within 0.15 atm of the exact answer to be graded correct. atm Thanation Check © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center I Q Search L³ ملةarrow_forward301.7 348.9 193.7 308.6 339.5 160.6 337.7 464.7 223.5 370.5 326.6 327.5 336.1 317.9 203.8 329.8 221.9 331.7 211.7 309.6 223.4 353.7 334.6 305.6 340.0 304.3 244.7 QUESTION: Using this group of data on regular tomato sauce concentration readings answer the following questions: 1. 95% Cl Confidence Interval (mmol/L) 2. [Na+] (mg/100 mL) 3. 95% Na+ Confidence Interval (mg/100 mL)arrow_forwardSearch Results Search Results Best Free Coursehero Unlo x b Success Confirmation of Q aleks.com/alekscgi/x/sl.exe/10_u-lgNslkr7j8P3jH-IQs_pBan HhvlTCeeBZbufu BYTIOHz7m7D3ZcHYUt80XL-5alyVpwDXM TEZayFYCav States of Matter Using a phase diagram to find a phase transition temperature or pressure Use the phase diagram of Substance X below to find the temperature at which X turns to a gas, if the pressure above the solid is 3.7 atm. pressure (atm) 0. 32- 16 solid liquid gas 200 temperature (K) Note: your answer must be within 20 °C of the exact answer to be graded correct. Дос Xarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning


General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning
General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY