
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN: 9781938168390
Author: Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher: OpenStax
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 14, Problem 106E
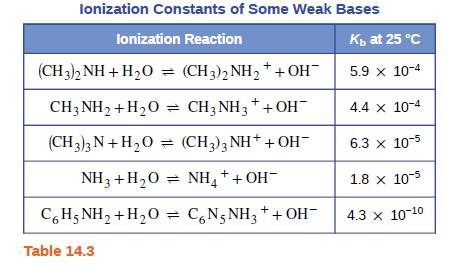
Which base in Table 14.3 is most appropriate for preparation of a buffer solution with a pH of 9.20? Explain your choice.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
What is the final product when hexanedioic acid reacts with 1º PCl5 and 2º NH3.
What is the final product when D-galactose reacts with hydroxylamine?
Indicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.
Chapter 14 Solutions
Chemistry by OpenStax (2015-05-04)
Ch. 14 - Write equations that show NH3 as both a conjugate...Ch. 14 - Write equations that show H2PO4- acting both as an...Ch. 14 - Show by suitable net ionic equations that each of...Ch. 14 - Show by suitable net ionic equations that each of...Ch. 14 - Show by suitable net ionic equations that each of...Ch. 14 - Show by suitable net ionic equations that each of...Ch. 14 - What is the conjugate acid of each of the...Ch. 14 - What is the conjugate acid of each of the...Ch. 14 - Identify and label the Bronsted-Lowry acid, its...Ch. 14 - Identify and label the Bronsted-Lowry acid, its...
Ch. 14 - What are amphiprotic species? Illustrate with...Ch. 14 - State which of the following species are...Ch. 14 - State which of the following species are...Ch. 14 - Is the self-ionization of water endothermic or...Ch. 14 - Explain why a sample of pure water at 40 C is...Ch. 14 - The ionization constant for water (Kw) is 2.91014...Ch. 14 - The ionization constant for water (Kw) is...Ch. 14 - Calculate the pH and the pOH of each of the...Ch. 14 - Calculate the pH and the pOH of each of the...Ch. 14 - What are the pH and pOH of a solution of 2.0 M...Ch. 14 - What are the hydronium and hydroxide ion...Ch. 14 - Calculate the hydrogen ion concentration and the...Ch. 14 - Calculate the hydronium ion concentration and the...Ch. 14 - The hydronium ion concentration in a sample of...Ch. 14 - The hydroxide ion concentration in household...Ch. 14 - Explain why the neutralization reaction of a...Ch. 14 - Explain why the neutralization reaction of a weak...Ch. 14 - Use this list of important industrial compounds...Ch. 14 - The odor of vinegar is due to the presence of...Ch. 14 - Household ammonia is a solution of the weak base...Ch. 14 - Explain why the ionization constant, Ka, for H2SO4...Ch. 14 - Explain why the ionization constant, Ka, for HI is...Ch. 14 - Gastric juice, the digestive ?uid produced in the...Ch. 14 - Nitric acid reacts with insoluble copper (II)...Ch. 14 - What is the ionization constant at 25 C for the...Ch. 14 - What is the ionization constant at 25 C for the...Ch. 14 - Which base, CH3NH2 or (CH3)2NH, is the stronger...Ch. 14 - Which is the stronger acid, NH4+ or HBrO?Ch. 14 - Which is the stronger base, (CH3)3N or H2BO3-?Ch. 14 - Predict which acid in each of the following pairs...Ch. 14 - Predict which compound in each of the following...Ch. 14 - Rank the compounds in each of the following groups...Ch. 14 - Rank the compounds in each of the following groups...Ch. 14 - Both HF and HCN ionize in water to a limited...Ch. 14 - The active ingredient formed by aspirin in the...Ch. 14 - What do we represent when we write:...Ch. 14 - Explain why equilibrium calculations are not...Ch. 14 - Are the concentrations of hydronium ion and...Ch. 14 - What two common assumptions can simplify...Ch. 14 - What two common assumptions can simplify...Ch. 14 - Which of the following will increase the percent...Ch. 14 - Which of the following will increase the percent...Ch. 14 - What is the effect on the concentrations of NO2-,...Ch. 14 - What is the effect on the concentration of...Ch. 14 - Why is the hydronium ion concentration in a...Ch. 14 - From the equilibrium concentrations given,...Ch. 14 - From the equilibrium concentrations given,...Ch. 14 - Determine Kb for the nitrite ion, NO2-. In a...Ch. 14 - Determine Ka for hydrogen sulfate ion, HSO4-. In a...Ch. 14 - Calculate the ionization constant for each of the...Ch. 14 - Calculate the ionization constant for each of the...Ch. 14 - For which of the following solutions must we...Ch. 14 - Even though both NH3 and C6H5NH2 are weak bases,...Ch. 14 - Calculate the equilibrium concentration of the...Ch. 14 - Calculate the equilibrium concentration of the...Ch. 14 - Calculate the equilibrium concentration of the...Ch. 14 - Calculate the equilibrium concentration of the...Ch. 14 - Using the Ka value of , place Al(H2O)63+ in the...Ch. 14 - Calculate the concentration of all solute species...Ch. 14 - Propionic acid, C2H5CO2H (Ka=1.34105), is used in...Ch. 14 - White vinegar is a 5.0% by mass solution of acetic...Ch. 14 - The ionization constant of lactic acid,...Ch. 14 - Nicotine, C10H14N2, is a base that will accept two...Ch. 14 - The pH of a 0.20-M solution of HP is 1.92....Ch. 14 - The pH of a 0.15-M solution of HSO4- is 1.43....Ch. 14 - The pH of a 0.10-M solution of caffeine is 11.16....Ch. 14 - Tile pH of a solution of household ammonia, a...Ch. 14 - Determine whether aqueous solutions of the...Ch. 14 - Determine whether aqueous solutions of the...Ch. 14 - Novocaine, C13H21O2N2Cl, is the salt of the base...Ch. 14 - Which of the following concentrations would be...Ch. 14 - Calculate the concentration of each species...Ch. 14 - Calculate the concentration of each species...Ch. 14 - Salicylic acid, HOC6H4CO2H, and its derivatives...Ch. 14 - The ion HTe- is an amphiprotic species; it can act...Ch. 14 - Explain why a buffer can be prepared from a...Ch. 14 - Explain why the pH does not change significantly...Ch. 14 - Explain why the pH does not change significantly...Ch. 14 - What is [H3O+] in a solution of 0.25 M CH3CO2H and...Ch. 14 - What is [H3O+] in a solution of 0.075 M HNO2 and...Ch. 14 - What is [OH-] in a solution of 0.125 M CH3NH2 and...Ch. 14 - What is [OH-] in a solution of 1.25 M NH3 and 0.78...Ch. 14 - What concentration of NH4NO3 is required to make...Ch. 14 - What concentration of NaF is required to make...Ch. 14 - What is the effect on the concentration of acetic...Ch. 14 - What is the effect on the concentration of...Ch. 14 - What will be the pH of a buffer solution prepared...Ch. 14 - Calculate the pH of a buffer solution prepared...Ch. 14 - How much solid NaCH3CO23H2O must be added to 0300...Ch. 14 - What mass of NH4Cl must be added to 0.750 L of a...Ch. 14 - A buffer solution is prepared from equal volumes...Ch. 14 - A 5.36-g sample of NH4Cl was added to 25.0 mL of...Ch. 14 - Which acid in Table 14.2 is most appropriate for...Ch. 14 - Which acid in Table 14.2 is most appropriate for...Ch. 14 - Which base in Table 14.3 is must appropriate for...Ch. 14 - Which base in Table 14.3 is most appropriate for...Ch. 14 - Saccharin, C7H4NSO3H, is a weak acid (Ka=2.1102)....Ch. 14 - What is the pH of 1.000 L of a solution of 100.0 g...Ch. 14 - Explain how to choose the appropriate acid-base...Ch. 14 - Explain why an acid-base indicator changes color...Ch. 14 - Why can we ignore the contribution of water to the...Ch. 14 - Why can we ignore the contribution of water to the...Ch. 14 - Draw a curve for a series of solutions of HF. Plot...Ch. 14 - Draw a curve similar to that shown in Figure 14.23...Ch. 14 - Calculate the pH at the following points in a...Ch. 14 - The indicator dinitrophenol is an acid with a Ka...
Additional Science Textbook Solutions
Find more solutions based on key concepts
21. The accompanying pedigree shows the transmission of a phenotypic character. Using B to represent a dominan...
Genetic Analysis: An Integrated Approach (3rd Edition)
CAUTION Why does the presence of extinct forms and transitional features in the fossil record support the patte...
Biological Science (6th Edition)
Raw Oysters and Antacids: A Deadly Mix? The highly acidic environment of the stomach kills most bacteria before...
Microbiology with Diseases by Body System (5th Edition)
In the following diagram, the white spheres represent hydrogen atoms and the blue Sphere represent the nitrogen...
Chemistry: The Central Science (14th Edition)
When you rub your cold hands together, the friction between them results in heat that warms your hands. Why doe...
Anatomy & Physiology (6th Edition)
Explain why 92% of 2,4-pemtanedione exists as the enol tautomer in hexane but only 15% of this compound exists ...
Organic Chemistry (8th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forwardQUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forward
- er your payment details | bar xb Home | bartleby x + aleksogi/x/isl.exe/1o u-lgNskr7j8P3jH-1Qs_pBanHhviTCeeBZbufuBYT0Hz7m7D3ZcW81NC1d8Kzb4srFik1OUFhKMUXzhGpw7k1 O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 1 3- 0- 0 200 Explanation Check temperature (K) 400 X Q Search L G 2025 McGraw Hill LLC. All Rights Reserved Terms of Use Privacy Cearrow_forward5.arrow_forward6.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning
General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY