
Concept explainers
PRACTICE PROBLEM 14.1
Provide a name for each of the following compounds.
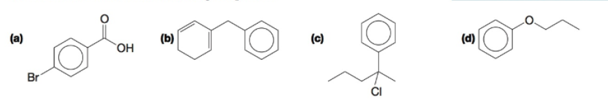
Interpretation: The name is to be provided for each of the given compound.
Concept Introduction:
The substituent and the benzene ring together give a common accepted parent name.
The position is indicated by the numbers, if more than one group is present on the benzene ring.
Benzene ring with large substituents are named by treating benzene as a substituent. In such cases, benzene as a substituent is named as a phenyl group.
The benzene ring is numbered such that the substituent will get the lowest possible number.
Answer to Problem 1PP
Solution:
a)
b)
c)
d) Propoxybenzene
Explanation of Solution
(a)
Given information:

The compound contains a common structural unit of benzoic acid and bromine as a substituent is present at the fourth position. Thus, the name of the compound is
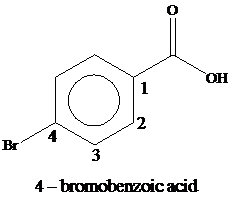
(b)
Given information:
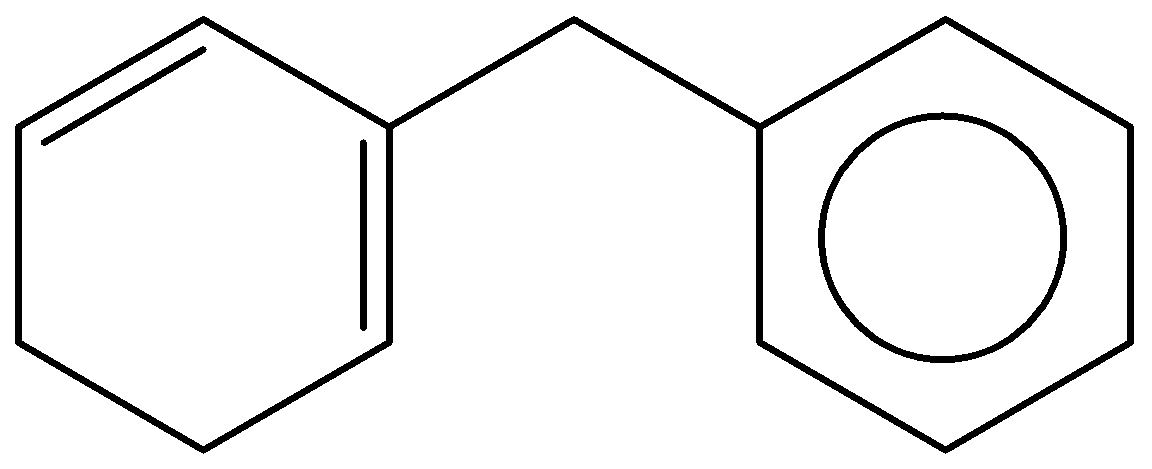
The compound contains a simple derivative of benzene named as benzyl group. In cyclohexadiene, two double bonds are present at the first and the third position, and benzyl group is present at the second position. Hence, the name of the compound is
(c)
Given information:

This compound contains five carbon atoms in its longest continuous chain. The base name is pentane. The numbering is done in such a way that the substituent gets the lowest number. There are two substituents in this structure-chloro and phenyl group. Both chloro group and phenyl group are located at the second carbon atom. So, the name of the compound is
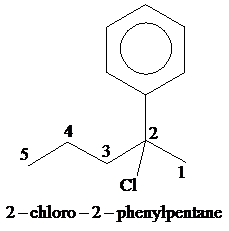
(d)
Given information:

The given compound contains ether as a functional group. The compound is named according to the groups bonded to the ether oxygen. The group bonded to the ether oxygen is phenyl and propyl. Hence, the name of the compound is Propoxybezene.
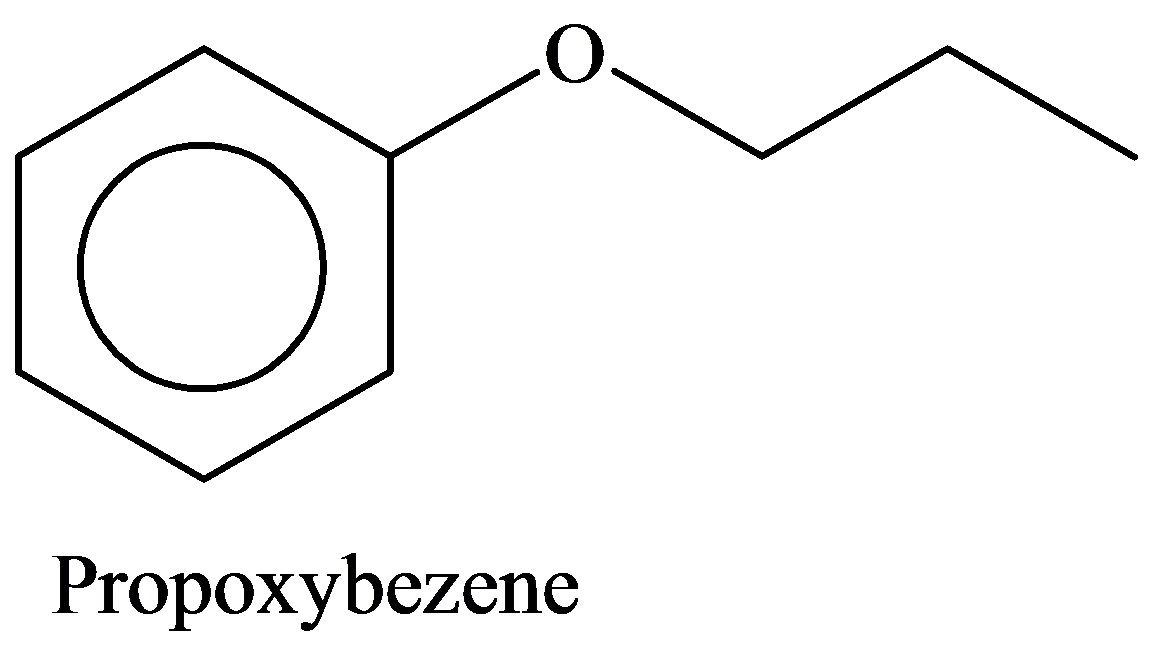
Want to see more full solutions like this?
Chapter 14 Solutions
Organic Chemistry, 12e Study Guide/Student Solutions Manual
Additional Science Textbook Solutions
Anatomy & Physiology (6th Edition)
Human Anatomy & Physiology (2nd Edition)
Campbell Biology (11th Edition)
Living By Chemistry: First Edition Textbook
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Chemistry: The Central Science (14th Edition)
- Show work. don't give Ai generated solutionarrow_forwardIn intercalation compounds, their sheets can be neutral or have a negative or positive charge, depending on the nature of the incorporated species and its structure. Is this statement correct?arrow_forwardThis thermodynamic cycle describes the formation of an ionic compound MX2 from a metal element M and nonmetal element X in their standard states. What is the lattice enthalpy of MX2 ? What is the enthalpy formation of MX2 ? Suppose both the heat of sublimation of M and the ionization enthalpy of M were smaller. Would MX2 be more stable? Or less? or impossible to tell without more information?arrow_forward
- I need to make 25mL of solution with the stocks shown below. How would I calculate the math?arrow_forwardWe are practicing calculating for making solutions. How would I calculate this?arrow_forwardBr. , H+ .OH Mg ether solvent H+, H₂O 17. Which one of the compounds below is the final product of the reaction sequence shown above? HO A HO HO OH D B OH HO OH C OH HO OH Earrow_forward
- 8:57 PM Sun Jan 26 Content ← Explanation Page X Content X ALEKS Jade Nicol - Le A https://www-av C www-awa.aleks.com O States of Matter Understanding consequences of important physical properties of liquids ? QUESTION Liquid A is known to have a lower viscosity and lower surface tension than Liquid B. Use these facts to predict the result of each experiment in the table below, if you can. experiment Liquid A and Liquid B are each pumped through tubes with an inside diameter of 27.0 mm, and the pressures PA and PB needed to produce a steady flow of 2.4 mL/s are measured. 25.0 mL of Liquid A are poured into a beaker, and 25.0 mL of Liquid B are poured into an identical beaker. Stirrers in each beaker are connected to motors, and the forces FA and FB needed to stir each liquid at a constant rate are measured. predicted outcome OPA will be greater than PB OPA will be less than PB OPA will be equal to PB It's impossible to predict whether PA or PB will be greater without more information.…arrow_forwardShow work. Don't give Ai generated solutionarrow_forward5. Please draw in the blanks the missing transition states and the correlated products. Explicitly display relevant absolute stereochemical configuration. MeOH I OMe H Endo transition state, dienophile approaching from the bottom of diene + H ཎྞཾ ཌཱརཱ༔,_o OMe H H OMe Endo transition state, dienophile approaching from the top of diene or from the bottom but horizontally flipped (draw one) + Exo transition state, dienophile approaching from the top of diene or from the bottom but horizontally flipped (draw one) Exo transition state, dienophile approaching from the top of diene or from the bottom but horizontally flipped (draw one) MeO H H MeO H MeO H MeO H Harrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
