
Concept explainers
Answers to all problems are at the end οΓthis book. Detailed solutions are available in the Student Solutions Manual. Study Guide, and Problems Book.
Characterizing a Covalent Enzyme Inhibitor Tosyl-L-phenylalanme cfaloromethyl
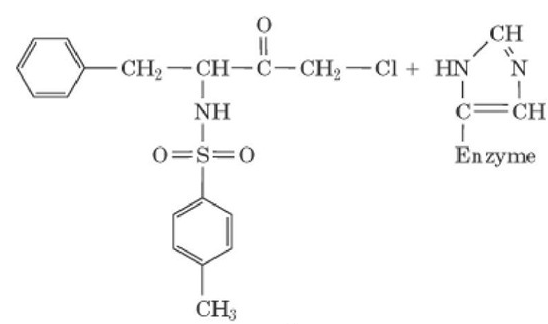
- Propose a mechanism for the inactivation reaction, indicating the structure of the produce(s).
- State why this inhibitor is specific tor cJiymotrypsin.
- Propose a reagent based on the structure of TPCK that might be an effective inhibitor of trypsin.
a.
To propose: The mechanism for the inactivation reaction which indicates the structure of the products.
Introduction:
Covalent enzyme inhibitor like TPCK is slowly reversible or totally irreversible. In many cases, these inhibitors are used to validate the pathway or target. There are targeted covalent enzyme inhibitors that have a slow offset, high binding efficiency as well as high potency to bind, increased selectivity decreased propensity for target-based drug resistance and prolonged pharmacodynamic effect.
Explanation of Solution
Chymotrypsin undergoes covalent inactivation reaction by nucleophilic attack on the
So, the mechanism of inactivation reaction is given below:

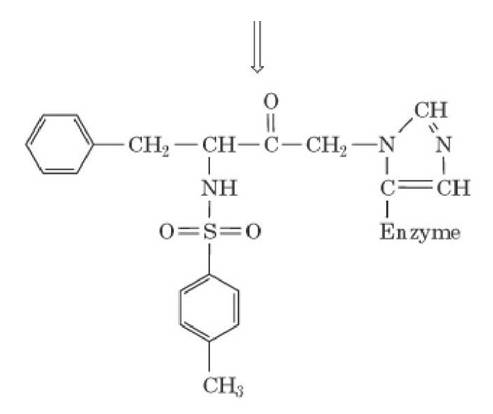
Hence, from the above discussion, it is clear that chymotrypsin undergoes covalent inhibition reaction.
b.
To propose: The reason for this inhibitor to be specific for chymotrypsin.
Introduction:
Covalent enzyme inhibitor like TPCK is slowly reversible or totally irreversible. In many cases, these inhibitors are used to validate the pathway or target. There are targeted covalent enzyme inhibitors that have a slow offset, high binding efficiency as well as high potency to bind, increased selectivity decreased propensity for target-based drug resistance and prolonged pharmacodynamic effects. Chymotrypsin plays an important part in the breakdown of proteins which is called proteolysis. The pancreas synthesizes the chymotrypsin. It cleaves the peptide amino bonds to digest the proteins.
Explanation of Solution
The inhibitor is chymotrypsin specific because the chloromethyl keto group reaches the active site by the hydrophobic reactions of chymotrypsin and the phenyl group of TPCK.
Hence, from the above discussion, it is clear that the inhibitor is chymotrypsin specific.
c.
To identify: A reagent that can be an effective inhibitor of Trypsin.
Introduction:
Chymotrypsin plays an important part in the breakdown of proteins which is called proteolysis. The pancreas synthesizes the chymotrypsin. It cleaves the peptide amino bonds to digest the proteins.
Explanation of Solution
The phenylalanine residue of TPCK has to be replaced with lysine or arginine to produce the specific reagents for the enzyme Trypsin.
Hence, from the above discussion, it is clear that the residual portion of TPCK only produces the specific reagent for Trypsin.
Want to see more full solutions like this?
Chapter 14 Solutions
Biochemistry
- Do schwann cells produce or act as myelin in the peripheral nervous system? I know that they encase and wrap around axons, but where does the myelin come into play?arrow_forwardThe enzyme lactate dehydrogenase (LDH) catalyzes the conversion of pyruvate to lactatein skeletal muscle cells using NAD/NADH during anaerobic “balanced” fermentation.Answer the following questions about this reaction. (a) Write out the two reductive half reactions and indicate the E ̊' for each half reaction. Write out the full balanced reaction for the pyruvate to lactate rxn and indicate the ∆E ̊' for the reaction. (b) What is the free energy change under standard state conditions for thisreaction? Which direction is spontaneous?(c) Assume that in skeletal muscle cells the ratio of [NAD+] to [NADH] is 100, and that the[pyruvate] = 0.40 mM and [lactate] = 4.0 mM. What is the free energy change (∆G')for the conversion of pyruvate to lactate? Indicate the direction in which the reactionis spontaneous under these cellular conditions.arrow_forwardWhy did the authors worry about the temperature-dependent solubility of the carriers in thebilayer? How did the authors determine whether the effect of freezing the lipid bilayer wasto decrease the solubility of the carriers (nonactin and valinomycin) or whether the effectwas to impair their ability to diffuse through the membrane (decrease their mobility)?arrow_forward
- Kranse et. al. measured the temperature dependence of conductance using membranescontaining the phospholipids glyceryl dipalmitate and glyceryl distearate. Describe themodifications in membrane content that you would employ to: (a) shift the temperature of the phase transition (b) make the ion conductance curve for valinomycin andnonactin more like that of gramicidinarrow_forwardObtain the sequence for the 5-HT receptor HTR1A and generate a hydropathy plot usingthe ExPASY tool ProtScale, the appropriate window, and the Kyte-Doolittle weightingalgorithm. How many transmembrane domains are present in this receptor? Attach yourhydropathy plot to your assignment.arrow_forwardCompare and contrast the structural features of the ion carrier valinomycin with those of achannel former like gramicidin. How does structural information help explain the mechanismby which these molecules conduct ions across membranes?arrow_forward
- A typical integral membrane protein has a stretch (or stretches) of ~20 hydrophobic aminoacids that form an α-helix that spans the bilayer (as is found in membrane proteins such asglycophorin A and bacteriorhodopsin). Compare and contrast the molecular and structural features of gramicidin with a membrane-spanning α-helix. Explain how gramicidin can forman ion channel when a typical membrane-spanning α-helix cannot (eg, glycophorin A).arrow_forwardThe titration curve of alanine shows the ionization of two functional groups with pK values of 2.34 and 9.69, corresponding to the ionization of the carboxyl and the protonated amino groups, respectively. The titration of di-, tri-, and larger oligopeptides of alanine also shows the ionization of only two functional groups, although the experimental pK values are different. The table summarizes the trend in pK values. Amino acid or peptide Ala Ala-Ala pKj pk₂ 2.34 9.69 3.12 8.30 Ala-Ala-Ala 3.39 8.03 Ala-(Ala)-Ala, n ≥ 4 3.42 7.94 Modify the molecules to show the oligopeptide Ala-Ala-Ala. You can modify the molecules by moving, adding, deleting, or changing atoms, bonds, or charges. C Select c Draw Templates More H с N 0 S Cl H H | | || H CH3 H CH, H CH₂ Complete the statements about the the pK, values of the Ala-Ala-Ala oligopeptide. The pK₁ value of 3.39 is associated with the -COO group of Ala-Ala-Ala. The pK2 value of 8.03 is associated with the -NH group of Ala-Ala-Ala. Erase Q2 Q…arrow_forwardFacts from the bacterium mals and to dept kan apa in a peptide with antidic properties. This peptide complex with the call membrance of other hacterial species, leading in bacterial death The structure of the peptide has been determined from (a) Cmplete acid hydes of the peptide, followed by amino acid analys, yielded quiar anunt of Lan, Om, Pfx, Prxa, and Wall Cmtiti, an amino acid od prosentin pockets but present in some peptides. Com has the tracture H *H,N-CH-CH-CH, -C- COO (b) The weight of the peptide in approximately 1,200 Th (c) The peptide failed to undergo hydrolysis when treated with the Hydrolysis of the carbonyl-terminal residue of a polypeptide une "NH, the year. This call there Pro or the police does not contain a froz (d) Treatment of the peptide with 1-haw-2,4-dicherer (11N1), followed by complete hydrolysis and ched only from and the derivative NO, Н ON NHCHI CH, CH, C coo +NH, (Hint: The 2,4-diphenyl derivative involves the amino group of a side chain rather than the…arrow_forward
 BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
