
Interpretation:
The cubane,
Concept introduction:
The complex vibrations exhibit by the polyatomic molecule is known as normal modes of vibrations. The vibrational modes of a molecule are IR or Raman active. If a molecule has centre of symmetry, then the modes which are IR-active will be Raman inactive and the modes that are IR-inactive will be Raman active. The total number of vibrational degrees of freedom for nonlinear molecule is represented by
Answer to Problem 14.82E
The cubane,
Explanation of Solution
The structure of cubane is shown below.
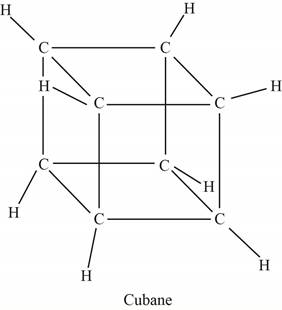
Figure 1
The point group of cubane is
The character table for point group
The expression
Similarly, the value of
The expression
The positive sign is taken for the proper rotation and negative sign is taken for improper axis.
Substitute the value of
Similarly, the value of
The expression
Substitute the value of
Similarly, the value of
The expression
The positive sign is taken for the proper rotation and negative sign is taken for improper axis.
Substitute the value of
Similarly, the value of
The expression
The positive sign is taken for the proper rotation and negative sign is taken for improper axis.
Substitute the value of
Similarly, the value of
The great orthogonality theorem for the reducible representation can be represented as,
Where,
•
•
•
•
•
The term
The irreducible representation for point group
Substitute the
Therefore, the cubane,
The cubane,
Want to see more full solutions like this?
Chapter 14 Solutions
Physical Chemistry
- H H:O::::H H H HH H::O:D:D:H HH HH H:O:D:D:H .. HH H:O:D:D:H H H Select the correct Lewis dot structure for the following compound: CH3CH2OHarrow_forwardRank the following compounds in order of decreasing boiling point. ннннн -С-С-Н . н-с- ННННН H ΗΤΗ НННН TTTĪ н-с-с-с-с-о-н НННН НН C' Н н-с-с-с-с-н НН || Ш НННН H-C-C-C-C-N-H ННННН IVarrow_forwardRank the following compounds in order of decreasing dipole moment. |>||>||| ||>|||>| |>|||>|| |||>||>| O ||>>||| H F H F H c=c || H c=c F F IIIarrow_forward
- choose the description that best describes the geometry for the following charged species ch3-arrow_forwardWhy isn't the ketone in this compound converted to an acetal or hemiacetal by the alcohol and acid?arrow_forwardWhat is the approximate bond angle around the nitrogen atom? HNH H Harrow_forward
- OH 1. NaOCH2CH3 Q 2. CH3CH2Br (1 equiv) H3O+ Select to Draw 1. NaOCH2 CH3 2. CH3Br (1 equiv) heat Select to Edit Select to Drawarrow_forwardComplete and balance the following half-reaction in acidic solution. Be sure to include the proper phases for all species within the reaction. S₂O₃²⁻(aq) → S₄O₆²⁻(aq)arrow_forwardQ Select to Edit NH3 (CH3)2CHCI (1 equiv) AICI 3 Select to Draw cat. H2SO4 SO3 (1 equiv) HO SOCl2 pyridine Select to Edit >arrow_forward
- Complete and balance the following half-reaction in basic solution. Be sure to include the proper phases for all species within the reaction. Zn(s) → Zn(OH)₄²⁻(aq)arrow_forwardb. ὋΗ CH3CH2OH H2SO4arrow_forwardFor the reaction A (g) → 3 B (g), Kp = 0.379 at 298 K. What is the value of ∆G for this reaction at 298 K when the partial pressures of A and B are 5.70 atm and 0.250 atm?arrow_forward
 Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning, Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning

