
Modified Mastering Chemistry with Pearson eText -- Standalone Access Card -- for Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
13th Edition
ISBN: 9780134562254
Author: Karen C Timberlake
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 14, Problem 14.53UTC
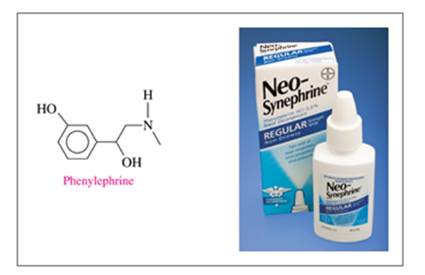
Phenylephrine is the active ingredient in some nose sprays used to reduce swelling of nasal membranes. Identify the
(14.1, 14.3, 14.5, 14.6)
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
You have started a patient on a new drug. Each dose introduces 40 pg/mL of drug after redistribution and prior to elimination. This drug is administered at 24 h intervals and has a half life of 24 h. What will the concentration of drug be after each of the first six doses? Show your work
a. What is the concentration after the first dose? in pg/mL
b. What is the concentration after the second dose? in pg/mL
c. What is the concentration after the third dose? in pg/mL
How many different molecules are drawn below?
Only 100% sure experts solve it correct complete solutions need to get full marks it's my quiz okkkk.take your time but solve full accurate okkk chemistry expert solve it.qno4
Chapter 14 Solutions
Modified Mastering Chemistry with Pearson eText -- Standalone Access Card -- for Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Ch. 14.1 - What carboxylic acid is responsible for the pain...Ch. 14.1 - What carboxylic acid is found in vinegar?Ch. 14.1 - Prob. 14.3PPCh. 14.1 - Prob. 14.4PPCh. 14.1 - Draw the condensed structural formulas for a and b...Ch. 14.1 - Draw the condensed structural formulas for a and b...Ch. 14.2 - Identify the compound in each group that is most...Ch. 14.2 - Prob. 14.8PPCh. 14.2 - Prob. 14.9PPCh. 14.2 - Prob. 14.10PP
Ch. 14.2 - Prob. 14.11PPCh. 14.2 - Prob. 14.12PPCh. 14.2 - Prob. 14.13PPCh. 14.2 - Prob. 14.14PPCh. 14.3 - Prob. 14.15PPCh. 14.3 - Prob. 14.16PPCh. 14.3 - Prob. 14.17PPCh. 14.3 - Prob. 14.18PPCh. 14.3 - Prob. 14.19PPCh. 14.3 - Prob. 14.20PPCh. 14.3 - Prob. 14.21PPCh. 14.3 - Prob. 14.22PPCh. 14.3 - Prob. 14.23PPCh. 14.3 - Prob. 14.24PPCh. 14.4 - What are the products of the acid hydrolysis of an...Ch. 14.4 - Prob. 14.26PPCh. 14.4 - Prob. 14.27PPCh. 14.4 - Prob. 14.28PPCh. 14.5 - Prob. 14.29PPCh. 14.5 - Prob. 14.30PPCh. 14.5 - Prob. 14.31PPCh. 14.5 - Prob. 14.32PPCh. 14.5 - Prob. 14.33PPCh. 14.5 - Prob. 14.34PPCh. 14.5 - Prob. 14.35PPCh. 14.5 - Prob. 14.36PPCh. 14.5 - Prob. 14.37PPCh. 14.5 - Prob. 14.38PPCh. 14.6 - Prob. 14.39PPCh. 14.6 - Prob. 14.40PPCh. 14.6 - Prob. 14.41PPCh. 14.6 - Prob. 14.42PPCh. 14.6 - Prob. 14.43PPCh. 14.6 - Prob. 14.44PPCh. 14.6 - Draw the condensed structural or line-angle...Ch. 14.6 - Draw the condensed structural or line-angle...Ch. 14.6 - a. Identify the functional groups in dicyclanil....Ch. 14.6 - a. Identify the functional groups in enrofloxacin....Ch. 14 - Prob. 14.49UTCCh. 14 - Prob. 14.50UTCCh. 14 - The ester methyl butanoate has the odor and flavor...Ch. 14 - Prob. 14.52UTCCh. 14 - Phenylephrine is the active ingredient in some...Ch. 14 - Melatonin is a naturally occurring compound in...Ch. 14 - Prob. 14.55UTCCh. 14 - Prob. 14.56UTCCh. 14 - Prob. 14.57APPCh. 14 - 14.58 Write the IUPAC and common names, if any,...Ch. 14 - Prob. 14.59APPCh. 14 - Prob. 14.60APPCh. 14 - Draw the condensed structural or line-angle...Ch. 14 - Prob. 14.62APPCh. 14 - Prob. 14.63APPCh. 14 - 14.64 Draw the condensed structural or line-angle...Ch. 14 - Prob. 14.65APPCh. 14 - 14.66 Write the common name and classify each of...Ch. 14 - Prob. 14.67APPCh. 14 - Draw the condensed structural or line-angle...Ch. 14 - Prob. 14.69APPCh. 14 - Prob. 14.70APPCh. 14 - Write the IUPAC name for each of the following:...Ch. 14 - Prob. 14.72APPCh. 14 - Prob. 14.73APPCh. 14 - Prob. 14.74APPCh. 14 - Prob. 14.75APPCh. 14 - Toradol is used in dentistry to relieve pain....Ch. 14 - Prob. 14.77CPCh. 14 - Draw the line-angle formula and write the IUPAC...Ch. 14 - Prob. 14.79CPCh. 14 - Prob. 14.80CPCh. 14 - Prob. 14.81CPCh. 14 - Prob. 14.82CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Nonearrow_forwardA complete tensile test was performed on a magnesium specimen of 12 mm diameter and 30 mm length, until breaking. The specimen is assumed to maintain a constant volume. Calculate the approximate value of the actual stress at breaking. TABLE. The tensile force F and the length of the specimen are represented for each L until breaking. F/N L/mm 0 30,0000 30,0296 5000 10000 30,0592 15000 30,0888 20000 30,15 25000 30,51 26500 30,90 27000 31,50 26500 32,10 25000 32,79arrow_forwardNonearrow_forward
- Differentiate between plastic deformation, elastic deformation, viscoelastic deformation and viscoplastic deformation.arrow_forward1.57 Draw all reasonable resonance structures for the following cation. Then draw the resonance hybrid.arrow_forwardFor the two questions below, draw the mechanism and form the major product.arrow_forward
- Indicate similarities and differences between natural, exchanged and pillared clays.arrow_forwardShow work. don't give Ai generated solutionarrow_forwardIn intercalation compounds, their sheets can be neutral or have a negative or positive charge, depending on the nature of the incorporated species and its structure. Is this statement correct?arrow_forward
- This thermodynamic cycle describes the formation of an ionic compound MX2 from a metal element M and nonmetal element X in their standard states. What is the lattice enthalpy of MX2 ? What is the enthalpy formation of MX2 ? Suppose both the heat of sublimation of M and the ionization enthalpy of M were smaller. Would MX2 be more stable? Or less? or impossible to tell without more information?arrow_forward7. Draw the mechanism to describe the following transformation: Note: This is a base catalyzed reaction. So, the last steps must make [OH]- OH [OH]¯ OH Heat Oarrow_forwardShow work with explanation...don't give Ai generated solutionarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning
Lipids - Fatty Acids, Triglycerides, Phospholipids, Terpenes, Waxes, Eicosanoids; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=7dmoH5dAvpY;License: Standard YouTube License, CC-BY