
Bundle: Chemistry For Today: General, Organic, And Biochemistry, 9th + Owlv2 With Mindtap Reader, 1 Term (6 Months) Printed Access Card
9th Edition
ISBN: 9781337580632
Author: Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 14, Problem 14.47E
The following compounds are cyclic acetals or ketals. Write structural formulas
for the hydrolysis products.
a.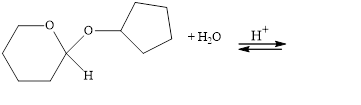
b.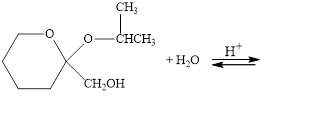
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
(ME EX1) Prblm #9/10
Can you explain in detail (step by step) I'm so confused with these problems. For turmber 13 can u turn them into lewis dot structures so I can better understand because, and then as well explain the resonance structure part. Thanks for the help.
Problems 19 and 20: (ME EX1) Can you please explain the following in detail? I'm having trouble understanding them. Both problems are difficult for me to explain in detail, so please include the drawings and answers.
(ME EX1) Prblm #4-11 Can you please help me and explain these I'm very confused in detail please. Prblm number 9 I don't understand at all (its soo confusing to me and redraw it so I can better depict it).
Chapter 14 Solutions
Bundle: Chemistry For Today: General, Organic, And Biochemistry, 9th + Owlv2 With Mindtap Reader, 1 Term (6 Months) Printed Access Card
Ch. 14 - Prob. 14.1ECh. 14 - Prob. 14.2ECh. 14 - Identify each of the following compounds as an...Ch. 14 - Identify each of the following compounds as an...Ch. 14 - Prob. 14.5ECh. 14 - Prob. 14.6ECh. 14 - Prob. 14.7ECh. 14 - Prob. 14.8ECh. 14 - Draw structural formulas and give IUPAC names for...Ch. 14 - Draw structural formulas and give IUPAC names for...
Ch. 14 - Each of the following names is wrong. Give the...Ch. 14 - Each of the following names is wrong. Give the...Ch. 14 - Prob. 14.13ECh. 14 - Prob. 14.14ECh. 14 - Prob. 14.15ECh. 14 - Explain why propane boils at 42C, whereas ethanal,...Ch. 14 - Use a dotted line to show hydrogen bonding between...Ch. 14 - Use a dotted line to show hydrogen bonding between...Ch. 14 - Prob. 14.19ECh. 14 - Prob. 14.20ECh. 14 - Prob. 14.21ECh. 14 - Prob. 14.22ECh. 14 - Prob. 14.23ECh. 14 - Prob. 14.24ECh. 14 - Label each of the following as acetals, ketals, or...Ch. 14 - Label each of the following as acetals, ketals, or...Ch. 14 - Label each of the following structures as a cyclic...Ch. 14 - Label each of the following structures as a...Ch. 14 - What two functional groups react to form the...Ch. 14 - Hemiacetals are sometimes referred to as potential...Ch. 14 - Complete the following statements: a. Oxidation of...Ch. 14 - Prob. 14.32ECh. 14 - Prob. 14.33ECh. 14 - Prob. 14.34ECh. 14 - Prob. 14.35ECh. 14 - Not all aldehyde give a positve Bendicts test....Ch. 14 - A stockroom assistant prepares three bottles, each...Ch. 14 - Glucose, the sugar present within the blood, gives...Ch. 14 - Fructose, present with glucose in honey, reacts...Ch. 14 - Prob. 14.40ECh. 14 - Prob. 14.41ECh. 14 - Complete the following equations. If no reaction...Ch. 14 - Complete the following equations. If no reaction...Ch. 14 - Describe the products that result when hydrogen...Ch. 14 - Prob. 14.45ECh. 14 - Draw structural formulas for the products of the...Ch. 14 - The following compounds are cyclic acetals or...Ch. 14 - The following compounds are cyclic acetals or...Ch. 14 - Write equations to show how the following...Ch. 14 - Prob. 14.50ECh. 14 - Identify the most important aldehyde and ketone...Ch. 14 - Using Table 14.3, name an aldehyde or ketone used...Ch. 14 - Prob. 14.53ECh. 14 - CH3COH(O)CH3COOHacetaldehydeaceticacid You need to...Ch. 14 - The addition of water to aldehydes and ketones...Ch. 14 - Prob. 14.56ECh. 14 - Formaldehyde levels above 0.10mg/1000L of ambient...Ch. 14 - In the IUPAC name for the following ketone, it is...Ch. 14 - Why can formaldehyde (CH2O) be prepared in the...Ch. 14 - Other addition reactions of aldehydes occur....Ch. 14 - Prob. 14.61ECh. 14 - Prob. 14.62ECh. 14 - Vanilla flavoring is either extracted from a...Ch. 14 - Prob. 14.64ECh. 14 - The use of acetone in laboratory experiments must...Ch. 14 - Prob. 14.66ECh. 14 - Prob. 14.67ECh. 14 - Which of the following would be classified as a...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- ME EX1) Prblm #19-20 I'm so confused with these problems. Can you please help me solve them and explain them? Problems number 19-20, and thanks! step by step and in detail for me please helparrow_forwardCalculate the flux of oxygen between the ocean and the atmosphere, given that: Temp = 18°C Salinity = 35 ppt Density = 1025 kg/m3 Oxygen concentration measured in bulk water = 263.84 mmol/m3 Wind speed = 7.4 m/s Oxygen is observed to be about 10% initially supersaturatedarrow_forward( ME EX1) Prblm 27-28: Can you explain to me both prblms in detail and for prblm 28 what do you mean bi conjugated bi ponds and those structures I'm confused...arrow_forward
- A. Determine the number of electrons in a system of cyclic conjugation (zero if no cyclic conjugation). B. Specify whether the species is "a"-aromatic, "aa"-anti-aromatic, or "na"-non-aromatic (neither aromatic nor anti-aromatic). (Presume rings to be planar unless structure obviously prevents planarity. If there is more than one conjugated ring, count electrons in the largest.) 1. A.Electrons in a cyclic conjugated system. 18 B.The compound is (a, aa, or na) a 2. A.Electrons in a cyclic conjugated system. 10 B.The compound is (a, aa, or na) naarrow_forwardWater is boiling at 1 atm pressure in a stainless steel pan on an electric range. It is observed that 2 kg of liquid water evaporates in 30 min. Find the rate of heat transfer to the water (kW).arrow_forwardCould you please turn this into a complete Lewis dot structure formula for me so I can visualize it more clearly? and then do the explaining for the resonance structures that were given please.arrow_forward
- Could you please turn this into a complete Lewis dot structure formula for me so I can visualize it more clearly? and then do the explaining for the question.arrow_forwardplease solve. If the answer is "no error" and it asks me to type something, and i typed a-helix, its always wrong.arrow_forwardCan you please solve and explain this for me in a simple way? I cant seem to comprehend this problem.arrow_forward
- Part I. Problem solving. Include all necessary calculations 13 provide plots and graphs. Complexation wl diphenyl carbazide (OPC) in acidic media is another type of sensitive photometric method used for the analysis of aqueous. hexavalent chromium. At 540nm the cherry-red complex as a result of DPC reaction w/ chromium can be photometrically measured. at this wavelength. - a 25mL The UV-vis analysis for the determination of nexavalent chromium in ground water sample is given below. The experiment was based on external calibration method w/ each measurement sample prepared are as follows lab sample analysis contained the standard 100 ppb croy cor groundwater sample, volumes used as indicated below), 12.50 mL of 0.02 M H2Soy and 5.50 ml of 100 ppm DPC (wi water to adjust final volume to 25-ml). The main stripping method was square wave voltammetry, following the conditions set in the main ASV experiment. Standard 100 Volumetric Groundwater H2SO4 0.20 M, flask Sample, mL ppb CrO4*, 100…arrow_forwardplease helparrow_forwardPredict the products of the following reactions. Draw mechanism arrows for each step for a, b, and c. a.) HBr b.) HI H₂O H2SO4 d.) C12 HO H2SO4 1.) BH3 2.) H2O2, NaOHarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER
07 Physical Properties of Organic Compounds; Author: Mindset;https://www.youtube.com/watch?v=UjlSgwq4w6U;License: Standard YouTube License, CC-BY