
Concept explainers
a.
Interpretation:
Chiral centers in the given monosaccharide has to be labelled, the monosaccharide has to be classified as D or L, the enantiomer of the given monosaccharide has to be drawn and also the Fischer projection formula has to be given.
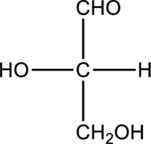
Concept Introduction:
A carbon atom that is bonded to four different groups is known as a chiral carbon atom. This can rotate the plane polarized light. D- and L- isomers of monosaccharide can be identified by looking into the chiral center that is farther from the carbonyl group. In a Fischer projection, if the
Enantiomers are two stereoisomers of a compound that rotate the plane polarized light exactly opposite. The configuration present in the enantiomers will be exactly opposite to each other.
Fischer projection formula simply uses cross for representing tetrahedral carbon atom. The carbon atom that is present in the intersection point in cross. The horizontal bonds means they are coming forward and they are present on wedge bond. The vertical bonds means they are pointing away and they are present on dashed lines.
b.
Interpretation:
Chiral centers in the given monosaccharide has to be labelled, the monosaccharide has to be classified as D or L, the enantiomer of the given monosaccharide has to be drawn and also the Fischer projection formula has to be given.
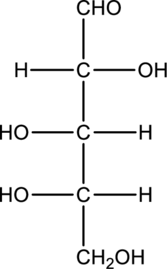
Concept Introduction:
Refer part “a.”.
c.
Interpretation:
Chiral centers in the given monosaccharide has to be labelled, the monosaccharide has to be classified as D or L, the enantiomer of the given monosaccharide has to be drawn and also the Fischer projection formula has to be given.
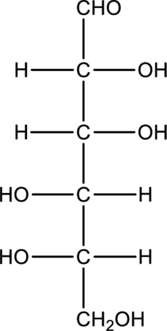
Concept Introduction:
Refer part “a.”.
Want to see the full answer?
Check out a sample textbook solution
Chapter 14 Solutions
Principles of General Organic & Biological Chemistry
- Use diagram to answer the following: 1.Is the overall rxn endo- or exothermic. Explain briefly your answer____________________2. How many steps in this mechanism?_____________3. Which is the rate determining step? Explain briefly your answer____________________4. Identify (circle and label) the reactants,the products and intermediate (Is a Cation, Anion, or a Radical?) Please explain and provide full understanding.arrow_forwardDraw the entire mechanism and add Curved Arrows to show clearly how electrons areredistributed in the process. Please explain and provide steps clearly.arrow_forward15) Create Lewis structure Br Brarrow_forward
- LIOT S How would you make 200. mL of a 0.5 M solution of CuSO4 5H2O from solid copper (II) sulfate? View Rubricarrow_forwardSteps and explantions pleasearrow_forwardMatch the denticity to the ligand. Water monodentate ✓ C₂O2 bidentate H₂NCH₂NHCH2NH2 bidentate x EDTA hexadentate Question 12 Partially correct Mark 2 out of 2 Flag question Provide the required information for the coordination compound shown below: Na NC-Ag-CN] Number of ligands: 20 Coordination number: 2✔ Geometry: linear Oxidation state of transition metal ion: +3 x in 12 correct out of 2 question Provide the required information for the coordination compound shown below. Na NC-Ag-CN] Number of ligands: 20 Coordination number: 2 Geometry: linear 0 Oxidation state of transition metal ion: +3Xarrow_forward
- Can you explain step by step behind what the synthetic strategy would be?arrow_forwardPlease explain step by step in detail the reasoning behind this problem/approach/and answer. thank you!arrow_forward2. Predict the product(s) that forms and explain why it forms. Assume that any necessary catalytic acid is present. .OH HO H₂N OHarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning





