
Concept explainers
Write structural formulas for the following compounds.
- a. ethyl isopropyl ether
- b. di-n-butyl ether
- c. 2-ethoxyoctane
- d. divinyl ether
- e. allyl methyl ether
- f. cyclohexene oxide
- g. cis-2,3-epoxyhexane
- h. (2R, 3S)-2-methoxypentan-3-ol
- i. trans-2,3-dimethyloxirane
(a)
Interpretation:
The structure formula for the given compound is to be drawn.
Concept introduction:
An organic compound where an oxygen atom is attached to two alkyl or aryl groups is known as ether. The general formula of ethers is
Answer to Problem 14.29SP
The structure of ethyl isopropyl ether is shown in Figure 1.
Explanation of Solution
The given compound is ethyl isopropyl ether. The root name isopropyl signifies three carbon atoms in a chain. Ethyl and isopropyl are the groups attached to an oxygen atom. The structure of ethyl isopropyl ether is shown below.
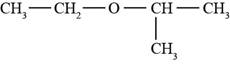
Figure 1
(b)
Interpretation:
The structure formula for the given compound is to be drawn.
Concept introduction:
An organic compound where an oxygen atom is attached to two alkyl or aryl groups is known as ether. The general formula of ethers is
Answer to Problem 14.29SP
The structure of di-n-butyl ether is shown in Figure 2.
Explanation of Solution
The given compound is di-n-butyl ether. The root name butyl signifies four carbon atoms in a chain and di-n-butyl indicates that two butyl groups are attached to an oxygen atom. The structure of di-n-butyl ether is shown below.
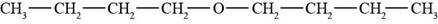
Figure 2
(c)
Interpretation:
The structure formula for the given compound is to be drawn.
Concept introduction:
An organic compound where an oxygen atom is attached to two alkyl or aryl groups is known as ether. The general formula of ethers is
Answer to Problem 14.29SP
The structure of 2-ethoxyoctane is shown in Figure 3.
Explanation of Solution
The given compound is 2-ethoxyoctane. The root octane signifies eight carbon atoms in a chain and 2-ethoxy shows that ethoxy group is present at second carbon atom of a chain. The structure of 2-ethoxyoctane is shown below.
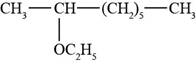
Figure 3
(d)
Interpretation:
The structure formula for the given compound is to be drawn.
Concept introduction:
An organic compound where an oxygen atom is attached to two alkyl or aryl groups is known as ether. The general formula of ethers is
Answer to Problem 14.29SP
The structure of divinyl ether is shown in Figure 4.
Explanation of Solution
The given compound is divinyl ether. The Vinyl group is the functional group having a formula
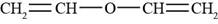
Figure 4
(e)
Interpretation:
The structure formula for the given compound is to be drawn.
Concept introduction:
An organic compound where an oxygen atom is attached to two alkyl or aryl groups is known as ether. The general formula of ethers is
Answer to Problem 14.29SP
The structure of allyl methyl ether is shown in Figure 5.
Explanation of Solution
The given compound is allyl methyl ether. An allyl group is used as a substituent and has a formula of
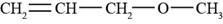
Figure 5
(f)
Interpretation:
The structure formula for the given compound is to be drawn.
Concept introduction:
An organic compound where an oxygen atom is attached to two alkyl or aryl groups is known as ether. The general formula of ethers is
Answer to Problem 14.29SP
The structure of cyclohexene oxide is shown in Figure 6.
Explanation of Solution
The given compound is cyclohexene oxide. The root name cyclohexene signifies six carbon atoms in a ring and oxide indicated the presence of epoxide ring. The structure of cyclohexene oxide is shown below.
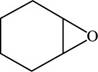
Figure 6
(g)
Interpretation:
The structure formula for the given compound is to be drawn.
Concept introduction:
An organic compound where an oxygen atom is attached to two alkyl or aryl groups is known as ether. The general formula of ethers is
Answer to Problem 14.29SP
The structure of
Explanation of Solution
The given compound is
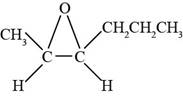
Figure 7
(h)
Interpretation:
The structure formula for the given compound is to be drawn.
Concept introduction:
An organic compound where an oxygen atom is attached to two alkyl or aryl groups is known as ether. The general formula of ethers is
Answer to Problem 14.29SP
The structure of
Explanation of Solution
The given compound is
According to Fisher projection,
For
The structure of
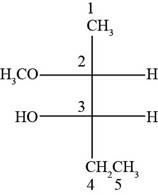
Figure 8
(i)
Interpretation:
The structure formula for the given compound is to be drawn.
Concept introduction:
An organic compound where an oxygen atom is attached to two alkyl or aryl groups is known as ether. The general formula of ethers is
Answer to Problem 14.29SP
The structure of
Explanation of Solution
The given compound is
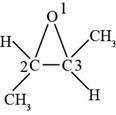
Figure 9
Want to see more full solutions like this?
Chapter 14 Solutions
Organic Chemistry (9th Edition)
- Q1: For each molecule, assign each stereocenter as R or S. Circle the meso compounds. Label each compound as chiral or achiral. OH HO CI Br H CI CI Br CI CI Xf x f g Br D OH Br Br H₂N R. IN Ill I -N S OMe D II H CO₂H 1/111 DuckDuckGarrow_forwardThese are synthesis questions. You need to show how the starting material can be converted into the product(s) shown. You may use any reactions we have learned. Show all the reagents you need. Show each molecule synthesized along the way and be sure to pay attention to the regiochemistry and stereochemistry preferences for each reaction. If a racemic molecule is made along the way, you need to draw both enantiomers and label the mixture as "racemic". All of the carbon atoms of the products must come from the starting material! ? H Harrow_forwardQ5: Draw every stereoisomer for 1-bromo-2-chloro-1,2-difluorocyclopentane. Clearly show stereochemistry by drawing the wedge-and-dashed bonds. Describe the relationship between each pair of the stereoisomers you have drawn.arrow_forward
- Classify each pair of molecules according to whether or not they can participate in hydrogen bonding with one another. Participate in hydrogen bonding CH3COCH3 and CH3COCH2CH3 H2O and (CH3CH2)2CO CH3COCH3 and CH₂ CHO Answer Bank Do not participate in hydrogen bonding CH3CH2OH and HCHO CH3COCH2CH3 and CH3OHarrow_forwardNonearrow_forwardQ4: Comparing (3S,4S)-3,4-dimethylhexane and (3R,4S)-3,4-dimethylhexane, which one is optically active? Briefly explain.arrow_forward
- Nonearrow_forwardNonearrow_forwardGiven the standard enthalpies of formation for the following substances, determine the reaction enthalpy for the following reaction. 4A (g) + 2B (g) → 2C (g) + 7D (g) AHrxn =?kJ Substance AH in kJ/mol A (g) - 20.42 B (g) + 32.18 C (g) - 72.51 D (g) - 17.87arrow_forward
- Determine ASran for Zn(s) + 2HCl(aq) = ZnCl2(aq) + H2(aq) given the following information: Standard Entropy Values of Various Substance Substance So (J/mol • K) 60.9 Zn(s) HCl(aq) 56.5 130.58 H2(g) Zn2+(aq) -106.5 55.10 CI (aq)arrow_forward3) Catalytic hydrogenation of the compound below produced the expected product. However, a byproduct with molecular formula C10H12O is also formed in small quantities. What is the by product?arrow_forwardWhat is the ΔHorxn of the reaction? NaOH(aq) + HCl(aq) → H2O(l) + NaCl(aq) ΔHorxn 1= ________ kJ/molarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





