
CHEMISTRY THE CENTRAL SCIENCE >EBOOK<
14th Edition
ISBN: 9780136873891
Author: Brown
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 14, Problem 110AE
The following mechanism has been proposed for the gas-phase reaction of chloroform (CHCI3) and chlorine:
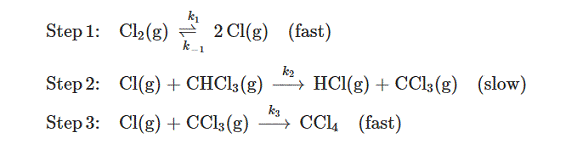
- What is the overall reaction? (b) What are the intermediates in the mechanism? (c) What is the molecularity of each of the elementary reactions? (b) What is the-determing step? (e) What is the rate law predicted by this mechanism? (Hint: The overall reaction order is not an integer.)
Expert Solution & Answer
Learn your wayIncludes step-by-step video

schedule12:14
Students have asked these similar questions
Determine whether the following reaction is an example of a nucleophilic substitution reaction:
H
NO2
H+
NO 2
+
Molecule A
Molecule B
Is this a nucleophilic substitution reaction?
If this is a nucleophilic substitution reaction, answer the remaining questions in this table.
What word or two-word phrase is used to describe the role Molecule A plays in this reaction?
What word or two-word phrase is used to describe the role Molecule B plays in this reaction?
Use a 6 + symbol to label the electrophilic carbon that is attacked during the substitution.
Highlight the leaving group on the appropriate reactant.
O Yes
○ No
☐
0
dx
000
HE
?
Draw the major organic product of the
Bronsted acid-base reaction.
Include all lone pairs and charges as
appropriate. Ignore any counterions.
:0:
NaOH
H
5. Calculate the total amount of heat transferred as 50 g of wat Specific heat H₂O (g) 2.00 J/g°C
-10 °C.
Specific heat H₂O (1)
Specific heat H₂O (s)
4.18 J/g°C
2.11 J/g°C
Heat of vaporization
2260 J/g
Heat of fusion
334 J/g
Melting point
0°C
6. Calculate the total amount of heat transferred as 25 g of
water is heated from 50 °C to 100 °C as a gas.
Boiling point
100 °C
Chapter 14 Solutions
CHEMISTRY THE CENTRAL SCIENCE >EBOOK<
Ch. 14.2 - If the experiment in Figure 14.2 is run for 60 s,...Ch. 14.2 - Prob. 14.1.2PECh. 14.2 - Which of the following could be the instantaneous...Ch. 14.2 - Using Figure 14.3, determine the instantaneous...Ch. 14.2 - At a certain time in a reaction, substance A is...Ch. 14.2 - Prob. 14.3.2PECh. 14.3 - Suppose the rate law for the reaction in this...Ch. 14.3 - Assuming that rate = k[A][B], rank the mixtures...Ch. 14.3 - Prob. 14.5.1PECh. 14.3 - Prob. 14.5.2PE
Ch. 14.3 - Consider the reaction examined above in the Sample...Ch. 14.3 - The following data were measured for the reaction...Ch. 14.4 - At 25 ° C, the decomposition of dinitrogen...Ch. 14.4 - Practice Exercise 2 The decomposition of dimethyl...Ch. 14.4 - Practice Exercise 1 For a certain reaction A ...Ch. 14.4 - Prob. 14.8.2PECh. 14.4 - Practice Exercise 1 We noted in an earlier...Ch. 14.4 - Practice Exercise 2 Using Equation 14.17,...Ch. 14.5 - Practice Exercise 1 This of the following change...Ch. 14.5 - Practice Exercise 2 Rank the rate constants of the...Ch. 14.5 - Practice Exercise 1 Using the data in Sample...Ch. 14.5 - Practice Exercise 2 To one significant figure,...Ch. 14.6 - Practice Exercise 1 Consider the following...Ch. 14.6 - For the reaction Mo(CO)6 +P(CH3)3 Mo(CO)5P(CH3)3...Ch. 14.6 - Practice Exercise 1 Consider the following...Ch. 14.6 - Practice Exercise 2 Consider the following...Ch. 14.6 - Practice Exercise 1 An Alternative two-step...Ch. 14.6 - Prob. 14.14.2PECh. 14.6 - Practice Exercise 1
Consider the...Ch. 14.6 - Prob. 14.15.2PECh. 14 - Prob. 1DECh. 14 - An automotive fuel injector dispenses a fine spray...Ch. 14 - Consider the following graph of the concentration...Ch. 14 - You study the rate of a reaction, measuring both...Ch. 14 - Suppose that for the reaction K+L M, you monitor...Ch. 14 - Prob. 5ECh. 14 - A friend studies a first-order reaction and...Ch. 14 - Prob. 7ECh. 14 - Which of the following linear plots do you expect...Ch. 14 - Prob. 9ECh. 14 - Prob. 10ECh. 14 - The following graph shows two different reaction...Ch. 14 - Prob. 12ECh. 14 - Prob. 13ECh. 14 - Draw a possible transition state for the...Ch. 14 - The following diagram represents an imaginary...Ch. 14 - 14.16 Draw a graph showing the reaction pathway...Ch. 14 - Prob. 17ECh. 14 - 14.18 (a) what are the units usually used to...Ch. 14 - Prob. 19ECh. 14 - A flask is charged with 0.100 mol of A and allowed...Ch. 14 - The isomerization of methyl isontrile (CH3NC) to...Ch. 14 - Prob. 22ECh. 14 - Prob. 23ECh. 14 - For each of the following gas-phase reactions,...Ch. 14 - (a) Consider the combustion of hydrogen, 2H2 (g) +...Ch. 14 - Prob. 26ECh. 14 - A reaction A+B C obeys the following rate law:...Ch. 14 - Prob. 28ECh. 14 - 14.29 The decomposition reaction of N2O5 in carbon...Ch. 14 - Prob. 30ECh. 14 - Prob. 31ECh. 14 - The reaction between ethyl bromide (C2H5Br) and...Ch. 14 - Prob. 33ECh. 14 - The reaction 2ClO2 (aq) + 2OH- (aq) ClO3- (aq) +...Ch. 14 - The following data were measured for the reaction...Ch. 14 - The following data were collected for the rate of...Ch. 14 - Consider the gas-phase reaction between nitric...Ch. 14 - Prob. 38ECh. 14 - Prob. 39ECh. 14 - Prob. 40ECh. 14 - Prob. 41ECh. 14 - Molecular iodine, I2 (g), dissociates into iodine...Ch. 14 - Prob. 43ECh. 14 - Prob. 44ECh. 14 - The reaction SO2Cl2 (g) O2 (g) + Cl2 (g) is first...Ch. 14 - Prob. 46ECh. 14 - Prob. 47ECh. 14 - Prob. 48ECh. 14 - Prob. 49ECh. 14 - Prob. 50ECh. 14 - (a) what factors determine whether a collision...Ch. 14 - (a) in which of the following reactions you expect...Ch. 14 - Calculate the fraction of atoms in a sample of...Ch. 14 - (a) the activation energy for the isomerization of...Ch. 14 - The gas-phase reaction CL (g) + HBr (g) + HCl (g)...Ch. 14 - Prob. 56ECh. 14 - Indicate whether each statement is true or false....Ch. 14 - Indicate whether each statement is true or false....Ch. 14 - Based on their activation energies and energy...Ch. 14 - Prob. 60ECh. 14 - Prob. 61ECh. 14 - Prob. 62ECh. 14 - The rate of the reaction CH3COOC2H5 (aq) + OH- ...Ch. 14 - Prob. 64ECh. 14 - Prob. 65ECh. 14 - Prob. 66ECh. 14 - What is the molecularity of each of the following...Ch. 14 - Prob. 68ECh. 14 - (a) based on the following reaction profile, how...Ch. 14 - Prob. 70ECh. 14 - Prob. 71ECh. 14 - Prob. 72ECh. 14 - The reaction 2NO (g) + CL2 (g) 2NOCl (g) was...Ch. 14 - You have studied the gas-phase oxidation of HBr by...Ch. 14 - Prob. 75ECh. 14 - Prob. 76ECh. 14 - Prob. 77ECh. 14 - Prob. 78ECh. 14 - Prob. 79ECh. 14 - The addition of No accelerates the decomposition...Ch. 14 - 14.81b Many metallic catalysts, particularly the...Ch. 14 - Prob. 82ECh. 14 - When D2 reacts with ethylene (C2H4) in the...Ch. 14 - Prob. 84ECh. 14 - Prob. 85ECh. 14 - The enzyme urease catalyzez the reaction of urea,(...Ch. 14 - Prob. 87ECh. 14 - Prob. 88ECh. 14 - Prob. 89AECh. 14 - Prob. 90AECh. 14 - Prob. 91AECh. 14 - Prob. 92AECh. 14 - Prob. 93AECh. 14 - Prob. 94AECh. 14 - Prob. 95AECh. 14 - Prob. 96AECh. 14 - [14.97]A first order reaction A B has the rate...Ch. 14 - Prob. 98AECh. 14 - Prob. 99AECh. 14 - Prob. 100AECh. 14 - Prob. 101AECh. 14 - Prob. 102AECh. 14 - Cyclopentadiene (C5H6) reacts with itself to form...Ch. 14 - Prob. 104AECh. 14 - At 280C, raw milk sours in 4.0 h but takes 48 h to...Ch. 14 - Prob. 106AECh. 14 - Prob. 107AECh. 14 - Prob. 108AECh. 14 - Prob. 109AECh. 14 - The following mechanism has been proposed for the...Ch. 14 - Prob. 111AECh. 14 - Prob. 112AECh. 14 - Platinum nanoparticles of diameter ~2 nm are...Ch. 14 - 14.114 One of the many remarkable enzymes in the...Ch. 14 - 14.115N Suppose that, in the absence of catalyst,...Ch. 14 - Prob. 116AECh. 14 - Dinitrogen pentoxide (N2O5) decomposes in...Ch. 14 - The reaction between ethyl iodide and hydroxide...Ch. 14 - Prob. 119IECh. 14 - Prob. 120IECh. 14 - Prob. 121IECh. 14 - The rates of many atmospheric reactions are...Ch. 14 - Prob. 123IECh. 14 - Prob. 124IECh. 14 - Prob. 125IE
Additional Science Textbook Solutions
Find more solutions based on key concepts
What are four functions of connective tissue?
Anatomy & Physiology (6th Edition)
Write an equation that uses the products of photosynthesis as reactants and the reactants of photosynthesis as ...
Campbell Biology in Focus (2nd Edition)
Another cross in Drosophila involved the recessive, X-linked genes yellow (y), white (w), and cut (ct). A yello...
Concepts of Genetics (12th Edition)
DNA sequences in manv human genes are very similar lo the sequences of corresponding genes in chimpanzees. The ...
Campbell Biology (11th Edition)
2. What are the primary functions of the skeletal system?
Human Anatomy & Physiology (2nd Edition)
4. What five specific threats to biodiversity are described in this chapter? Provide an example of each.
Biology: Life on Earth (11th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Calculate the total amount of heat transferred as 50 g of Water -10°C. Calculate the total amount of heat transferred as 25 g of water is heated from 50°C to 100°C as a gas. \table[[Specific heat H₂O(g), 2.00°C Η 2 g 5. Calculate the total amount of heat transferred as 50 g of wat Specific heat H₂O (g) 2.00 J/g°C -10 °C. 4.18 J/g°C 2.11 J/g°C 2260 J/g 334 J/g Specific heat H₂O (1) Specific heat H₂O (s) Heat of vaporization Heat of fusion Melting point 6. Calculate the total amount of heat transferred as 25 g of water is heated from 50 °C to 100 °C as a gas. Boiling point 100 °C 0°Carrow_forwardWrite formulas for ionic compounds composed of the following ions. Use units as a guide to your solutions. 24. sodium and nitrate 25. calcium and chlorate 26. aluminum and carbonate 27. CHALLENGE Write the formula for an ionic compound formed by ions from a group 2 element and polyatomic ions composed of only carbon and oxygen.show work step by steparrow_forwardADDITIONAL PRACTICE PRACTICE Problems Write formulas for ionic compounds composed of the following ions. Use units as a guide to your solutions. 24. sodium and nitrate 25. calcium and chlorate 26. aluminum and carbonate 27. CHALLENGE Write the formula for an ionic compound formed by ions from a group 2 element and polyatomic ions composed of only carbon and oxygen. ounds 1998arrow_forward
- 7:35 < Dji Question 19 of 22 5G 50% Submit What is the pH of a buffer made from 0.350 mol of HBrO (Ka = 2.5 × 10-9) and 0.120 mol of KBRO in 2.0 L of solution? | 1 2 3 ☑ 4 5 6 C 7 8 ☐ 9 +/- Tap here for additional resources ||| 0 ×10 Гarrow_forwardaw the major substitution products you would expect for the reaction shown below. If substitution would not occur at a significant rate under these conditions, check the box underneath the drawing area instead. Be sure you use wedge and dash bonds where necessary, for example to distinguish between major products. Note for advanced students: you can assume that the reaction mixture is heated mildly, somewhat above room temperature, but strong heat or reflux is not used. B C Br HO O Substitution will not occur at a significant rate. Explanation Check + Х Click and drag to start drawing a structure. © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibarrow_forwardComplete the following reactions with the necessary reagents to complete the shown transformation. Example: 1. 2. ? 3. 018 Br OH Answer: H₂O, H2SO4, HgSO4arrow_forward
- 7:34 • < Question 18 of 22 5G 50% Submit What is the pH of a buffer made from 0.220 mol of HCNO (Ka = 3.5 × 10-4) and 0.410 mol of NaCNO in 2.0 L of solution? 1 2 3 ☑ 4 5 6 C 7 8 | 9 +/- 0 ×10 Tap here for additional resources ||| Гarrow_forward6:46 ✔ 5G 58% < Question 7 of 22 Submit What is the primary species in solution at the halfway point in a titration of NH3 with HBr? A NH3 and H+ B NH₁+ and H+ C NH4+ D NH3 and NH4+ Tap here for additional resources |||arrow_forward6:49 Dji < Question 15 of 22 4G 57% Submit The pOH of a solution is 10.50. What is the OH- concentration in the solution? A 3.2 × 10-4 M B C 3.2 x 10-11 M 10.50 M D 4.2 M E 3.50 M Tap here for additional resources |||arrow_forward
- ヨ 6:49 Dji < Question 13 of 22 5G 57% Submit The pH of a solution is 2.40. What is the H+ concentration in the solution? A B 2.5 x 10-12 M 4.0 × 10-3 M C 2.40 M D 4.76 M 11.60 M Tap here for additional resources |||arrow_forwardヨ C 6:48 Di✔ < Question 12 of 22 5G 57% Submit The pH of a solution is 12.50. What is the H+ concentration in the solution? A 0.032 M B 3.2 × 10-13 M 1.5 M D 9.25 M 12.50 M Tap here for additional resources |||arrow_forwardヨ C 6:48 Di✔ < Question 11 of 22 5G 57% Submit The pH of a solution is 1.50. What is the H+ concentration in the solution? A 0.032 M B 3.2 × 10-13 M 1.5 M D 2.15 M 12.50 M Tap here for additional resources |||arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Physical Chemistry
Chemistry
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Wadsworth Cengage Learning,

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning
Kinetics: Initial Rates and Integrated Rate Laws; Author: Professor Dave Explains;https://www.youtube.com/watch?v=wYqQCojggyM;License: Standard YouTube License, CC-BY