
Interpretation:
Fisher projections are of two type D stereoisomers and L stereoisomer. You have to decide a given fisher projection is either D stereoisomers or L stereoisomers.
Concept introduction:
The three- dimensional structure stereoisomers on a plane represents the Fisher projections.
In the Fischer projection, a chiral carbon represented as intersection of horizontal and vertical line.
The horizontal lines are shown by bonds pointing above the plane and vertical lines as bonds pointing below the plane.
In fisher projection, the carbonyl group of
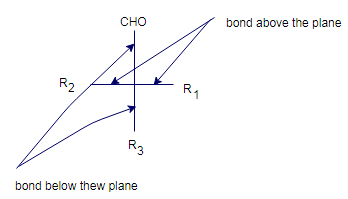
D and L classification based on the furthest chiral C from ketone or aldehyde.
D: if
L: it
Want to see the full answer?
Check out a sample textbook solution
Chapter 13 Solutions
Chemistry: An Introduction to General, Organic, and Biological Chemistry (12th Edition) - Standalone book
- Which representation(s) show polymer structures that are likely to result in rigid, hard materials and those that are likely to result in flexible, stretchable, soft materials?arrow_forward3. Enter the molecular weight of the product obtained from the Williamson Ether Synthesis? OH OH & OH excess CH3l Ag₂Oarrow_forwardPlease answer 1, 2 and 3 on the endarrow_forward
- In the box below, specify which of the given compounds are very soluble in polar aprotic solvents. You may select more than one compound. Choose one or more: NaCl NH4Cl CH3CH2CH2CH2CH2CN CH3CH2OH hexan-2-one NaOH CH3SCH3arrow_forwardOn the following structure, select all of the atoms that could ACCEPT a hydrogen bond. Ignore possible complications of aromaticity. When selecting be sure to click on the center of the atom.arrow_forwardRank the compounds below from lowest to highest melting point.arrow_forward
- 18 Question (1 point) Draw the line structure form of the given partially condensed structure in the box provided. :ÖH HC HC H2 ΙΩ Н2 CH2 CH3 CH3 partially condensed formarrow_forwardsomeone else has already submitted the same question on here and it was the incorrect answer.arrow_forwardThe reaction: 2NO2(g) ⇌ N2O4(g) is an exothermic reaction, ΔH=-58.0 kJ/molrxn at 0°C the KP is 58.If the initial partial pressures of both NO2(g) and N2O4(g) are 2.00 atm:A) Is the reaction at equilibrium? If not, what is the value of Q? B) Which direction will the reaction go to reach equilibrium? C) Use an ICE table to find the equilibrium pressures.arrow_forward
- The dissociation of the weak acid, nitrous acid, HNO2, takes place according to the reaction: HNO2 (aq) ⇌ H+(aq) + NO2–(aq) K=7.2 X 10-4 When 1.00 mole of HNO2 is added to 1.00 L of water, the H+ concentration at equilibrium is 0.0265 M.A) Calculate the value of Q if 1.00 L of water is added? B) How will reaction shift if 1.00 L of water is added?arrow_forwardSuppose a certain copolymer elastomeric material “styrene-butadiene rubber”) contains styrene ("S") monomers –(C8H8)– and butadiene ("B") monomers –(C4H6)– and that their numerical ratio S:B = 1:8. What is the mass ratio mS:mB of the two monomers in the material? What is the molecular mass M of a macromolecule of this copolymer with degree of polymerization n = 60,000? Data: AC = 12.01 u, AH = 1.008 u.arrow_forwardLab Questions from Lab: Gravimetric Determination of Calcium as CaC2O4•H2O What is the purpose of the methyl red indicator? Why does a color change to yellow tell you that the reaction is complete? Why is the precipitate rinsed with ice-cold water in step 4? Why not room temperature or hot water? Why is it important that the funnels be placed in a desiccator before weighing (steps 1 and 5)?arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





