
Chemistry: An Introduction to General, Organic, and Biological Chemistry (12th Edition) - Standalone book
12th Edition
ISBN: 9780321908445
Author: Karen C. Timberlake
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Question
Chapter 13, Problem 13.52UTC
Interpretation Introduction
Interpretation:
Chiral: Molecules that are non- superimposible mirror image, it has no symmetry either point of symmetry line of symmetry or plane of symmetry: It has a chiral carbon, chiral carbon is that carbon which is attached to our different group is called chiral carbon, or chiral center.
Achiral: Molecules that are superimposible of its mirror image. It has symmetry.
Concept introduction:
Simple rule to identify chiral center: It is that tetrahedral bonded carbon, where all four substituents are different. This carbon is also known as stereocenter.
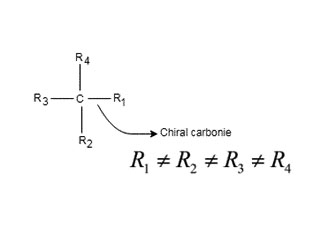
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Draw a structural formula for the major product of the acid-base reaction shown.
H
0
N
+
HCI (1 mole)
CH3
N'
(1 mole)
CH3
You do not have to consider stereochemistry.
●
•
Do not include counter-ions, e.g., Na+, I, in your answer.
. In those cases in which there are two reactants, draw only the product from
989
CH3
344
?
[F
Question 15
What is the major neutral organic product for the following sequence?
1.
POCI₂
pyridine
?
2. OsO4
OH
3. NaHSO
Major Organic Product
✓ OH
OH
'OH
OH
'OH
'CI
URGENT! PLEASE HELP!
Chapter 13 Solutions
Chemistry: An Introduction to General, Organic, and Biological Chemistry (12th Edition) - Standalone book
Ch. 13.1 - Prob. 13.1QAPCh. 13.1 - Prob. 13.2QAPCh. 13.1 - Prob. 13.3QAPCh. 13.1 - Prob. 13.4QAPCh. 13.1 - Prob. 13.5QAPCh. 13.1 - Prob. 13.6QAPCh. 13.1 - Prob. 13.7QAPCh. 13.1 - Prob. 13.8QAPCh. 13.1 - Prob. 13.9QAPCh. 13.1 - Prob. 13.10QAP
Ch. 13.2 - Prob. 13.11QAPCh. 13.2 - Prob. 13.12QAPCh. 13.2 - Identify the chiral carbon in each of the...Ch. 13.2 - Prob. 13.14QAPCh. 13.2 - Prob. 13.15QAPCh. 13.2 - Prob. 13.16QAPCh. 13.2 - Prob. 13.17QAPCh. 13.2 - Prob. 13.18QAPCh. 13.2 - Prob. 13.19QAPCh. 13.2 - Prob. 13.20QAPCh. 13.3 - Prob. 13.21QAPCh. 13.3 - Prob. 13.22QAPCh. 13.3 - Prob. 13.23QAPCh. 13.3 - Prob. 13.24QAPCh. 13.3 - Prob. 13.25QAPCh. 13.3 - Prob. 13.26QAPCh. 13.3 - Prob. 13.27QAPCh. 13.3 - Prob. 13.28QAPCh. 13.3 - Prob. 13.29QAPCh. 13.3 - Prob. 13.30QAPCh. 13.4 - What are the kind and number of atoms in the ring...Ch. 13.4 - What are the kind and number of atoms in the ring...Ch. 13.4 - Prob. 13.33QAPCh. 13.4 - Prob. 13.34QAPCh. 13.4 - Prob. 13.35QAPCh. 13.4 - Identify each of the following as the a or ß...Ch. 13.5 - Prob. 13.37QAPCh. 13.5 - Prob. 13.38QAPCh. 13.5 - Prob. 13.39QAPCh. 13.5 - Prob. 13.40QAPCh. 13.6 - Prob. 13.41QAPCh. 13.6 - Prob. 13.42QAPCh. 13.6 - Prob. 13.43QAPCh. 13.6 - Prob. 13.44QAPCh. 13.6 - Prob. 13.45QAPCh. 13.6 - Prob. 13.46QAPCh. 13.7 - Describe the similarities and differences in the...Ch. 13.7 - Prob. 13.48QAPCh. 13.7 - Prob. 13.49QAPCh. 13.7 - Prob. 13.50QAPCh. 13 - Prob. 13.51UTCCh. 13 - Prob. 13.52UTCCh. 13 - Prob. 13.53UTCCh. 13 - Prob. 13.54UTCCh. 13 - Prob. 13.55UTCCh. 13 - Prob. 13.56UTCCh. 13 - Prob. 13.57AQAPCh. 13 - Prob. 13.58AQAPCh. 13 - Prob. 13.59AQAPCh. 13 - Prob. 13.60AQAPCh. 13 - Prob. 13.61AQAPCh. 13 - Prob. 13.62AQAPCh. 13 - Prob. 13.63AQAPCh. 13 - Prob. 13.64AQAPCh. 13 - Prob. 13.65AQAPCh. 13 - Prob. 13.66AQAPCh. 13 - Prob. 13.67AQAPCh. 13 - Prob. 13.68AQAPCh. 13 - Prob. 13.69AQAPCh. 13 - Prob. 13.70AQAPCh. 13 - Prob. 13.71CQCh. 13 - Prob. 13.72CQCh. 13 - 13.77 Gentiobiose is found in saffron. (13.4,...Ch. 13 - Prob. 13.74CQ
Knowledge Booster
Similar questions
- Could you please solve the first problem in this way and present it similarly but color-coded or step by step so I can understand it better? Thank you!arrow_forwardCould you please solve the first problem in this way and present it similarly but (color-coded) and step by step so I can understand it better? Thank you! I want to see what they are doingarrow_forwardCan you please help mne with this problem. Im a visual person, so can you redraw it, potentislly color code and then as well explain it. I know im given CO2 use that to explain to me, as well as maybe give me a second example just to clarify even more with drawings (visuals) and explanations.arrow_forward
- Part 1. Aqueous 0.010M AgNO 3 is slowly added to a 50-ml solution containing both carbonate [co32-] = 0.105 M and sulfate [soy] = 0.164 M anions. Given the ksp of Ag2CO3 and Ag₂ soy below. Answer the ff: Ag₂ CO3 = 2 Ag+ caq) + co} (aq) ksp = 8.10 × 10-12 Ag₂SO4 = 2Ag+(aq) + soy² (aq) ksp = 1.20 × 10-5 a) which salt will precipitate first? (b) What % of the first anion precipitated will remain in the solution. by the time the second anion starts to precipitate? (c) What is the effect of low pH (more acidic) condition on the separate of the carbonate and sulfate anions via silver precipitation? What is the effect of high pH (more basic)? Provide appropriate explanation per answerarrow_forwardPart 4. Butanoic acid (ka= 1.52× 10-5) has a partition coefficient of 3.0 (favors benzene) when distributed bet. water and benzene. What is the formal concentration of butanoic acid in each phase when 0.10M aqueous butanoic acid is extracted w❘ 25 mL of benzene 100 mL of a) at pit 5.00 b) at pH 9.00arrow_forwardCalculate activation energy (Ea) from the following kinetic data: Temp (oC) Time (s) 23.0 180. 32.1 131 40.0 101 51.8 86.0 Group of answer choices 0.0269 kJ/mole 2610 kJ/mole 27.6 kJ/mole 0.215 kJ/mole 20.8 kJ/molearrow_forward
- Calculate activation energy (Ea) from the following kinetic data: Temp (oC) Time (s) 23.0 180. 32.1 131 40.0 101 51.8 86.0 choices: 0.0269 kJ/mole 2610 kJ/mole 27.6 kJ/mole 0.215 kJ/mole 20.8 kJ/molearrow_forwardCalculate activation energy (Ea) from the following kinetic data: Temp (oC) Time (s) 23.0 180. 32.1 131 40.0 101 51.8 86.0arrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY