
Modified Mastering Chemistry with Pearson eText -- Standalone Access Card -- for Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
13th Edition
ISBN: 9780134562254
Author: Karen C Timberlake
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 13.2, Problem 13.19PP
Identify the chiral carbon in each of the following compounds:
a. citronellol; one enantiomer has the odor of geranium
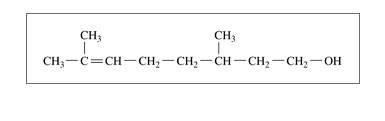
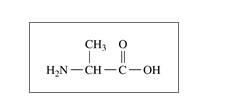
b. alanine,an amino acid
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
The complex anion in Ba₂[Cr(CN)6] is a tetragonally distorted octahedral complex (Dan). Baz[Cr(CN)6] is
paramagnetic at room temperature with S = 1. Assume that the complex is a low-spin complex.
a) Identify if the [Cr(CN)6] anionic complex has 4 long and
2 short bonds (left side of figure) or if the complex has 4
short and 2 long bonds (right side of figure) with respect
to Oh symmetry. Use crystal field theory to answer this
question. Explain/rationalize your decision. Can the
provided information decide on the order of orbital
energies?
Dah
Tetragonal Distortion
ய
Dab
z-compression
z-elongation
x and y elongation
O symmetry
x and y compression
E
eg d² dx²-y²
t2g
dxy dxz dyz
Question 4 a) continued:
Provide your explanations in the space below.
b) At low temperatures Ba₂[Cr(CN)6] is ferromagnetically ordered with a phase transition to a paramagnetic
phase at Tc = 150K. Sketch the magnetic susceptibility vs. temperature in the diagram below. Indicate Tc
as well as the paramagnetic and…
a) Draw the octahedral mer-[FeCl3(CN)3] complex and determine its point group. Use proper wedges and
dashes in order to illustrate 3 dimensional details. Use the point group to determine if the complex has a
resulting net dipole moment and describe its allowed direction with respect to its symmetry elements (if
applicable).
ード
M
4-
b) Substitute one chlorido ligand in mer-[FeCl3(CN)3] 4 with one fluorido ligand. Determine all possible
isomers and their corresponding point groups. Use the point groups to determine if the complexes have
resulting net dipole moments and describe their allowed direction with respect to its symmetry elements (if
applicable). The number of complex sketches below is not necessarily indicative of the number of isomers.
4-
4-
☐☐☐
c) Substitute two chlorido ligands in mer-[FeCl3 (CN)3] 4 with two fluorido ligands. Determine all possible
isomers and their corresponding point groups.. Use the point groups to determine if the complexes have
resulting net dipole…
Show work. don't give Ai generated solution
Chapter 13 Solutions
Modified Mastering Chemistry with Pearson eText -- Standalone Access Card -- for Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Ch. 13.1 - Prob. 13.1PPCh. 13.1 - Prob. 13.2PPCh. 13.1 - Prob. 13.3PPCh. 13.1 - Prob. 13.4PPCh. 13.1 - Prob. 13.5PPCh. 13.1 - Prob. 13.6PPCh. 13.1 - Prob. 13.7PPCh. 13.1 - Prob. 13.8PPCh. 13.1 - Prob. 13.9PPCh. 13.1 - Prob. 13.10PP
Ch. 13.2 - Prob. 13.11PPCh. 13.2 - Prob. 13.12PPCh. 13.2 - Prob. 13.13PPCh. 13.2 - Prob. 13.14PPCh. 13.2 - Prob. 13.15PPCh. 13.2 - Prob. 13.16PPCh. 13.2 - Identify each of the following as D or L: a. b. c.Ch. 13.2 - Identify each of the following as D or L: a. b. c.Ch. 13.2 - Identify the chiral carbon in each of the...Ch. 13.2 - Prob. 13.20PPCh. 13.3 - Prob. 13.21PPCh. 13.3 - Prob. 13.22PPCh. 13.3 - Prob. 13.23PPCh. 13.3 - Prob. 13.24PPCh. 13.3 - Prob. 13.25PPCh. 13.3 - Prob. 13.26PPCh. 13.3 - Prob. 13.27PPCh. 13.3 - Prob. 13.28PPCh. 13.3 - Prob. 13.29PPCh. 13.3 - Prob. 13.30PPCh. 13.4 - What are the kind and number of atoms in the ring...Ch. 13.4 - What are the kind and number of atoms in the ring...Ch. 13.4 - Prob. 13.33PPCh. 13.4 - Prob. 13.34PPCh. 13.4 - Prob. 13.35PPCh. 13.4 - Identify each of the following as the a or ß...Ch. 13.5 - Prob. 13.37PPCh. 13.5 - Prob. 13.38PPCh. 13.5 - Prob. 13.39PPCh. 13.5 - Prob. 13.40PPCh. 13.6 - Prob. 13.41PPCh. 13.6 - Prob. 13.42PPCh. 13.6 - Prob. 13.43PPCh. 13.6 - Prob. 13.44PPCh. 13.6 - Prob. 13.45PPCh. 13.6 - Prob. 13.46PPCh. 13.7 - Describe the similarities and differences in the...Ch. 13.7 - Prob. 13.48PPCh. 13.7 - Prob. 13.49PPCh. 13.7 - Prob. 13.50PPCh. 13.7 - Prob. 13.51PPCh. 13.7 - Prob. 13.52PPCh. 13.7 - Prob. 13.53PPCh. 13.7 - Prob. 13.54PPCh. 13 - Prob. 13.55UTCCh. 13 - Prob. 13.56UTCCh. 13 - Prob. 13.57UTCCh. 13 - Prob. 13.58UTCCh. 13 - Prob. 13.59UTCCh. 13 - Prob. 13.60UTCCh. 13 - Prob. 13.61APPCh. 13 - Prob. 13.62APPCh. 13 - Prob. 13.63APPCh. 13 - Prob. 13.64APPCh. 13 - Prob. 13.65APPCh. 13 - Prob. 13.66APPCh. 13 - Prob. 13.67APPCh. 13 - Prob. 13.68APPCh. 13 - Prob. 13.69APPCh. 13 - Prob. 13.70APPCh. 13 - Prob. 13.71APPCh. 13 - Prob. 13.72APPCh. 13 - Prob. 13.73APPCh. 13 - Prob. 13.74APPCh. 13 - Prob. 13.75CPCh. 13 - Prob. 13.76CPCh. 13 - 13.77 Gentiobiose is found in saffron. (13.4,...Ch. 13 - Prob. 13.78CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Differentiate electron spin and electron spin moment.arrow_forwardDifferentiate between nuclear spin and electron spin.arrow_forwardDraw the trigonal prismatic MH6 molecular compound, where M is a 3d transition metal. a) Draw the trigonal prismatic MH6 molecular compound and determine its point group. b) i. What is the symmetry species for the 4s orbital on the central metal? ii. What is the symmetry species for the 3dx²-y² orbital on the central metal? Note: The z-axis is the principal axis. iii. Suggest a crystal field energy diagram for a d² electron configuration in a trigonal prismatic coordination environment. Label the metal d-orbital with their corresponding symmetry species label. Use the appropriate character table in the resource section.arrow_forward
- this is an organic chemistry question please answer accordindly!! please post the solution draw the figures on a paper please hand drawn and post, please answer EACH part till the end and dont just provide wordy explanations, please draw them on a paper and post clearly!! answer the full question with all details EACH PART CLEARLY please thanks!! im reposting this please solve all parts and draw it not just word explanations!!arrow_forwardA mixture of 0.412 M C12, 0.544 M F2, and 0.843 M CIF is enclosed in a vessel and heated to 2500 K. C12(g) + F2(g )2CIF(g) Kc = 20.0 at 2500 K Calculate the equilibrium concentration of each gas at 2500 K. [C12] = M [F2] = M [ CIF] =arrow_forwardShow reaction mechanism with explanation. don't give Ai generated solutionarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning


General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning
CBSE Class 12 Chemistry || Polymers || Full Chapter || By Shiksha House; Author: Best for NEET;https://www.youtube.com/watch?v=OxdJlS0xZ0Y;License: Standard YouTube License, CC-BY