
Modified Mastering Chemistry with Pearson eText -- Standalone Access Card -- for Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
13th Edition
ISBN: 9780134562254
Author: Karen C Timberlake
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Question
Chapter 13.4, Problem 13.35PP
Interpretation Introduction
To identify: Each of the following as
a. 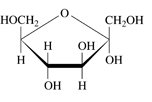
b.
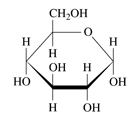
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
If the dissociation energy of one mole of O2 is 5.17 eV, determine the wavelength that must be used to dissociate it with electromagnetic radiation. Indicate how many Einstein's of this radiation are needed to dissociate 1 liter of O2 at 25°C and 1 atm of pressure.Data: 1 eV = 96485 kJ mol-1; R = 0.082 atm L K-1; c = 2.998x108 m s-1; h = 6.626x10-34 J s; NA = 6.022x 1023 mol-1
Indicate the number of Einsteins that are equivalent to 550 kJ mol⁻¹ of absorbed energy (wavelength 475 nm).
Indicate the number of einsteins that are equivalent to 550 kJ mol⁻¹ of absorbed energy?
Chapter 13 Solutions
Modified Mastering Chemistry with Pearson eText -- Standalone Access Card -- for Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Ch. 13.1 - Prob. 13.1PPCh. 13.1 - Prob. 13.2PPCh. 13.1 - Prob. 13.3PPCh. 13.1 - Prob. 13.4PPCh. 13.1 - Prob. 13.5PPCh. 13.1 - Prob. 13.6PPCh. 13.1 - Prob. 13.7PPCh. 13.1 - Prob. 13.8PPCh. 13.1 - Prob. 13.9PPCh. 13.1 - Prob. 13.10PP
Ch. 13.2 - Prob. 13.11PPCh. 13.2 - Prob. 13.12PPCh. 13.2 - Prob. 13.13PPCh. 13.2 - Prob. 13.14PPCh. 13.2 - Prob. 13.15PPCh. 13.2 - Prob. 13.16PPCh. 13.2 - Identify each of the following as D or L: a. b. c.Ch. 13.2 - Identify each of the following as D or L: a. b. c.Ch. 13.2 - Identify the chiral carbon in each of the...Ch. 13.2 - Prob. 13.20PPCh. 13.3 - Prob. 13.21PPCh. 13.3 - Prob. 13.22PPCh. 13.3 - Prob. 13.23PPCh. 13.3 - Prob. 13.24PPCh. 13.3 - Prob. 13.25PPCh. 13.3 - Prob. 13.26PPCh. 13.3 - Prob. 13.27PPCh. 13.3 - Prob. 13.28PPCh. 13.3 - Prob. 13.29PPCh. 13.3 - Prob. 13.30PPCh. 13.4 - What are the kind and number of atoms in the ring...Ch. 13.4 - What are the kind and number of atoms in the ring...Ch. 13.4 - Prob. 13.33PPCh. 13.4 - Prob. 13.34PPCh. 13.4 - Prob. 13.35PPCh. 13.4 - Identify each of the following as the a or ß...Ch. 13.5 - Prob. 13.37PPCh. 13.5 - Prob. 13.38PPCh. 13.5 - Prob. 13.39PPCh. 13.5 - Prob. 13.40PPCh. 13.6 - Prob. 13.41PPCh. 13.6 - Prob. 13.42PPCh. 13.6 - Prob. 13.43PPCh. 13.6 - Prob. 13.44PPCh. 13.6 - Prob. 13.45PPCh. 13.6 - Prob. 13.46PPCh. 13.7 - Describe the similarities and differences in the...Ch. 13.7 - Prob. 13.48PPCh. 13.7 - Prob. 13.49PPCh. 13.7 - Prob. 13.50PPCh. 13.7 - Prob. 13.51PPCh. 13.7 - Prob. 13.52PPCh. 13.7 - Prob. 13.53PPCh. 13.7 - Prob. 13.54PPCh. 13 - Prob. 13.55UTCCh. 13 - Prob. 13.56UTCCh. 13 - Prob. 13.57UTCCh. 13 - Prob. 13.58UTCCh. 13 - Prob. 13.59UTCCh. 13 - Prob. 13.60UTCCh. 13 - Prob. 13.61APPCh. 13 - Prob. 13.62APPCh. 13 - Prob. 13.63APPCh. 13 - Prob. 13.64APPCh. 13 - Prob. 13.65APPCh. 13 - Prob. 13.66APPCh. 13 - Prob. 13.67APPCh. 13 - Prob. 13.68APPCh. 13 - Prob. 13.69APPCh. 13 - Prob. 13.70APPCh. 13 - Prob. 13.71APPCh. 13 - Prob. 13.72APPCh. 13 - Prob. 13.73APPCh. 13 - Prob. 13.74APPCh. 13 - Prob. 13.75CPCh. 13 - Prob. 13.76CPCh. 13 - 13.77 Gentiobiose is found in saffron. (13.4,...Ch. 13 - Prob. 13.78CP
Knowledge Booster
Similar questions
- A unit used in photochemistry is the einstein. If 400 kJ mol-1 of energy has been absorbed, how many einsteins is this equivalent to?arrow_forwardFor the condensation reaction between Alanine and histidine write the amididation reaction mechanism using arrows then write the three letter code for the product of the reaction and the one letter code for the product of the reaction.arrow_forwardWrite the amididation reaction mechanism of p-aminophenol and acetic acid to produce acetaminophen please use arrows.arrow_forward
- Write the amididation reaction mechanism of a-aminophenol and acetic acid to produce acetaminophenarrow_forwardFor the condensation reaction between Alamine and histamine, please help me write the amididation reaction mechanism. Then write the three letter code for the product of the reaction, then write the one letter code for the product of the reaction. arrow_forwardHow to draw the reaction mechasnism belowarrow_forward
- Name the following molecules with IUpacarrow_forwardWhat is the molecular orbital for cyclopropenyl anion and is it aromatic, antiaromatic or nonaromatic?arrow_forwardUsing the chart describe the change from cystine to tyrosine and its impact on the protein. Using the chart describe the change from histidine to aspartic acid and its impact on the protein.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY