
Foundations of College Chemistry 15e Binder Ready Version + WileyPLUS Registration Card
15th Edition
ISBN: 9781119231318
Author: Morris Hein
Publisher: Wiley (WileyPLUS Products)
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 13, Problem 43AE
Interpretation Introduction
Interpretation:
Generalization from below graph about boiling point and molar mass has to be explained.
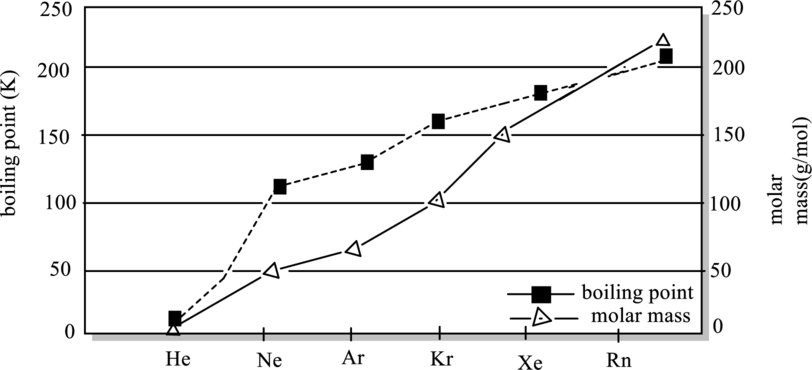
Concept Introduction:
Temperature at which vapor pressure becomes equal to the external pressure is termed as boiling point. At this point pressure of liquid becomes equal to the vapor pressure.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Provide the reasonable steps to achieve the following synthesis.
Identify which compound is more acidic. Justify your choice.
Provide the reasonable steps to achieve the following synthesis.
Chapter 13 Solutions
Foundations of College Chemistry 15e Binder Ready Version + WileyPLUS Registration Card
Ch. 13.2 - Prob. 13.1PCh. 13.2 - Prob. 13.2PCh. 13.3 - Prob. 13.3PCh. 13.3 - Prob. 13.4PCh. 13.4 - Prob. 13.5PCh. 13.5 - Prob. 13.6PCh. 13.5 - Prob. 13.7PCh. 13.5 - Prob. 13.8PCh. 13.6 - Prob. 13.9PCh. 13.6 - Prob. 13.10P
Ch. 13 - Prob. 1RQCh. 13 - Prob. 2RQCh. 13 - Prob. 3RQCh. 13 - Prob. 4RQCh. 13 - Prob. 5RQCh. 13 - Prob. 6RQCh. 13 - Prob. 7RQCh. 13 - Prob. 8RQCh. 13 - Prob. 9RQCh. 13 - Prob. 10RQCh. 13 - Prob. 11RQCh. 13 - Prob. 12RQCh. 13 - Prob. 13RQCh. 13 - Prob. 14RQCh. 13 - Prob. 15RQCh. 13 - Prob. 16RQCh. 13 - Prob. 17RQCh. 13 - Prob. 19RQCh. 13 - Prob. 20RQCh. 13 - Prob. 21RQCh. 13 - Prob. 22RQCh. 13 - Prob. 23RQCh. 13 - Prob. 24RQCh. 13 - Prob. 25RQCh. 13 - Prob. 26RQCh. 13 - Prob. 27RQCh. 13 - Prob. 28RQCh. 13 - Prob. 29RQCh. 13 - Prob. 30RQCh. 13 - Prob. 31RQCh. 13 - Prob. 32RQCh. 13 - Prob. 33RQCh. 13 - Prob. 34RQCh. 13 - Prob. 35RQCh. 13 - Prob. 36RQCh. 13 - Prob. 37RQCh. 13 - Prob. 38RQCh. 13 - Prob. 39RQCh. 13 - Prob. 40RQCh. 13 - Prob. 41RQCh. 13 - Prob. 42RQCh. 13 - Prob. 43RQCh. 13 - Prob. 1PECh. 13 - Prob. 2PECh. 13 - Prob. 3PECh. 13 - Prob. 4PECh. 13 - Prob. 5PECh. 13 - Prob. 6PECh. 13 - Prob. 7PECh. 13 - Prob. 8PECh. 13 - Prob. 9PECh. 13 - Prob. 10PECh. 13 - Prob. 11PECh. 13 - Prob. 12PECh. 13 - Prob. 13PECh. 13 - Prob. 14PECh. 13 - Prob. 15PECh. 13 - Prob. 16PECh. 13 - Prob. 17PECh. 13 - Prob. 18PECh. 13 - Prob. 19PECh. 13 - Prob. 20PECh. 13 - Prob. 21PECh. 13 - Prob. 22PECh. 13 - Prob. 23PECh. 13 - Prob. 24PECh. 13 - Prob. 25PECh. 13 - Prob. 26PECh. 13 - Prob. 27PECh. 13 - Prob. 28PECh. 13 - Prob. 29PECh. 13 - Prob. 30PECh. 13 - Prob. 31PECh. 13 - Prob. 32PECh. 13 - Prob. 33AECh. 13 - Prob. 34AECh. 13 - Prob. 35AECh. 13 - Prob. 36AECh. 13 - Prob. 38AECh. 13 - Prob. 39AECh. 13 - Prob. 40AECh. 13 - Prob. 41AECh. 13 - Prob. 42AECh. 13 - Prob. 43AECh. 13 - Prob. 44AECh. 13 - Prob. 45AECh. 13 - Prob. 46AECh. 13 - Prob. 47AECh. 13 - Prob. 48AECh. 13 - Prob. 49AECh. 13 - Prob. 50AECh. 13 - Prob. 51AECh. 13 - Prob. 52AECh. 13 - Prob. 53AECh. 13 - Prob. 54AECh. 13 - Prob. 55AECh. 13 - Prob. 56AECh. 13 - Prob. 57AECh. 13 - Prob. 58AECh. 13 - Prob. 59AECh. 13 - Prob. 60AECh. 13 - Prob. 61AECh. 13 - Prob. 62AECh. 13 - Prob. 63AECh. 13 - Prob. 64AECh. 13 - Prob. 65AECh. 13 - Prob. 66AECh. 13 - Prob. 67AECh. 13 - Prob. 69CECh. 13 - Prob. 70CECh. 13 - Prob. 71CECh. 13 - Prob. 72CE
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- When anisole is treated with excess bromine, the reaction gives a product which shows two singlets in 1H NMR. Draw the product.arrow_forward(ii) Draw a reasonable mechanism for the following reaction: CI NaOH heat OH (hint: SNAr Reaction) :arrow_forwardDraw the major product in each of the following reaction:arrow_forward
- Draw the mechanism for the following Friedel-Craft reaction. AlBr3 Brarrow_forward(a) Draw the structures of A and B in the following reaction. (i) NaNH2, NH3(1) A + B (ii) H3O+arrow_forwardFor the reaction 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 →> NO₂+ NO3_(K1) NO2 + NO3 → N2O5 (k-1) NO2 + NO3 → → NO2 + O2 + NO (K2) NO + N2O5- NO2 + NO2 + NO2 (K3) d[N₂O5] __2k‚k₂[N2O5] Indicate whether the following rate expression is acceptable: dt k₁₁+ k₂arrow_forward
- Consider the following decomposition reaction of N2O5(g): For the reaction 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 → NO2 + NO3 (K1) NO2 + NO3 → N2O5 (k-1) NO2 + NO3 → NO2 + O2 + NO (K2) NO + N2O5 → NO2 + NO2 + NO2 (K3) Indicate whether the following rate expression is acceptable: d[N2O5] = -k₁[N₂O₂] + K¸₁[NO₂][NO3] - K¸[NO₂]³ dtarrow_forwardIn a reaction of A + B to give C, another compound other than A, B or C may appear in the kinetic equation.arrow_forwardFor the reaction 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 →> NO₂+ NO3_(K1) NO2 + NO3 → N2O5 (k-1) NO2 + NO3 → → NO2 + O2 + NO (K2) NO + N2O5- NO2 + NO2 + NO2 (K3) d[N₂O5] __2k‚k₂[N2O5] Indicate whether the following rate expression is acceptable: dt k₁₁+ k₂arrow_forward
- Given the reaction R + Q → P, indicate the rate law with respect to R, with respect to P and with respect to P.arrow_forwardSteps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forwardk₁ Given the reaction A B, indicate k-1 d[A] (A). the rate law with respect to A: (B). the rate law with respect to B: d[B] dt dtarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

World of Chemistry
Chemistry
ISBN:9780618562763
Author:Steven S. Zumdahl
Publisher:Houghton Mifflin College Div

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning
Solutions: Crash Course Chemistry #27; Author: Crash Course;https://www.youtube.com/watch?v=9h2f1Bjr0p4;License: Standard YouTube License, CC-BY