
Concept explainers
(a)
Interpretation: The constitutional isomers formed by the monochlorination of given
Concept introduction: The compounds with same chemical formula but different arrangement of atoms in space are known as the constitutional isomers.
The reaction of halogen with alkane results in the formation of
Answer to Problem 27P
The five constitutional isomers formed by the reaction of
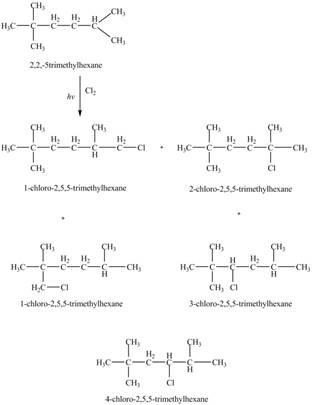
The four constitutional isomers formed by the reaction of
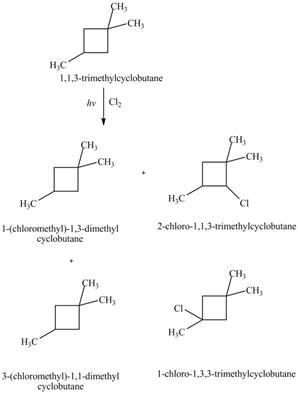
Explanation of Solution
The compounds with same chemical formula but have different arrangement of atoms in space is known as the constitutional isomer.
The reaction of halogen with alkane results in the formation of alkyl halide. This type of reaction is a radical substitution reaction. The halogenation of alkanes takes place via radical intermediates. If
The given alkanes are shown below.
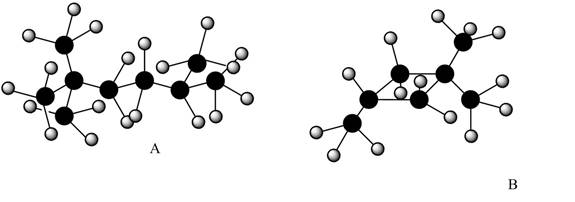
Figure 1
The given structure is in ball and stick model. The white color balls represent hydrogen atom and black color ball represents carbon atoms.
Therefore, structure of given alkanes are,
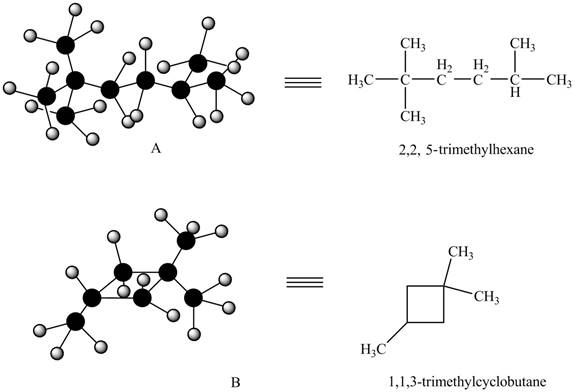
Figure 2
The constitutional isomers formed by the reaction of
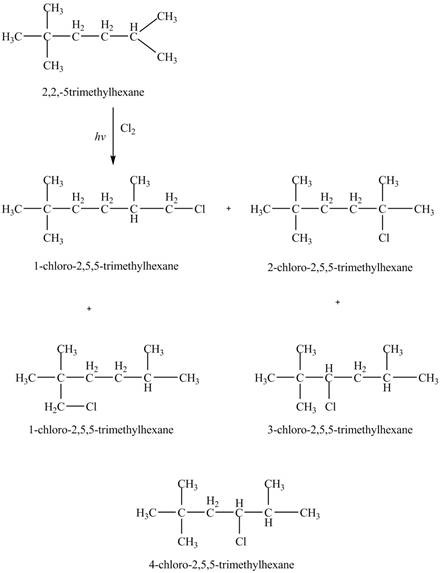
Figure 3
Therefore, the five constitutional isomers formed by the reaction of
Similarly, the constitutional isomers formed by the reaction of
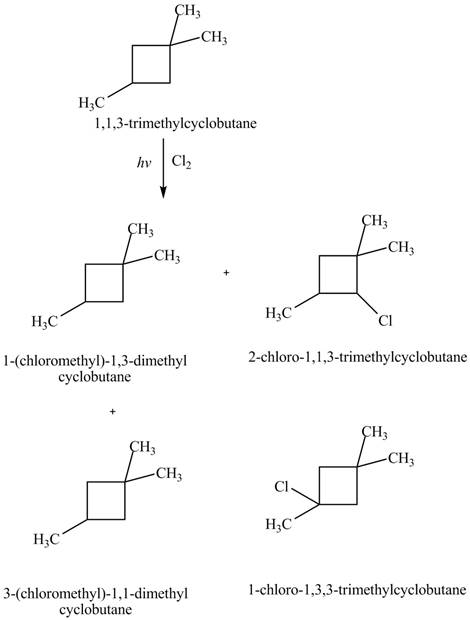
Figure 4
Therefore, the four constitutional isomers formed by the reaction of
The constitutional isomers formed by monochlorination of given alkanes with
(b)
Interpretation: The major bromination product formed by heating each alkane with
Concept introduction: The reaction of hydrogen halide with alkane results in the formation of alkyl halide. The halogenation of alkanes takes place via radical intermediates. If
Answer to Problem 27P
The major product formed by the reaction of
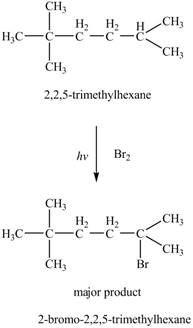
The major product formed by the reaction of
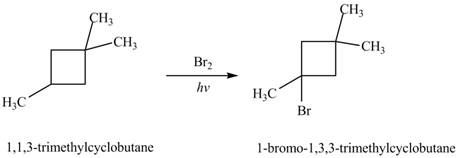
Explanation of Solution
The reaction of hydrogen halide with alkane results in the formation of alkyl halide. The halogenation of alkanes takes place via radical intermediates. If
The major product formed by the reaction of
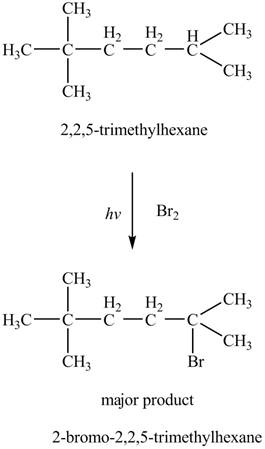
Figure 5
The major product formed by the reaction of

Figure 6
The major product formed by monochlorination of given alkanes with
Want to see more full solutions like this?
Chapter 13 Solutions
ORG CHEM CONNECT CARD
- Synthesize 2-Hydroxy-2-phenylacetonitrile from phenylmethanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- If possible, please provide the formula of the compound 3,3-dimethylbut-2-enal.arrow_forwardSynthesize 1,4-dibromobenzene from acetanilide (N-phenylacetamide) using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardIndicate the products obtained by mixing (3-oxo-3-phenylpropyl)triphenylphosphonium bromide with sodium hydride.arrow_forward
- We mix N-ethyl-2-hexanamine with excess methyl iodide and followed by heating with aqueous Ag2O. Indicate the major products obtained.arrow_forwardIndicate the products obtained by mixing acetophenone with iodine and NaOH.arrow_forwardIndicate the products obtained by mixing 2-Propanone and ethyllithium and performing a subsequent acid hydrolysis.arrow_forward
- Indicate the products obtained if (E)-2-butenal and 3-oxo-butanenitrile are mixed with sodium ethoxide in ethanol.arrow_forwardQuestion 3 (4 points), Draw a full arrow-pushing mechanism for the following reaction Please draw all structures clearly. Note that this intramolecular cyclization is analogous to the mechanism for halohydrin formation. COH Br + HBr Brarrow_forwardIndicate the products obtained if 2,2-dimethylpropanal and acetaldehyde are mixed with sodium ethoxide in ethanol.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





