
Concept explainers
Draw the enol tautomers for each of the following compounds. If the compound has more than one enol tautomer, indicate which one is more stable.
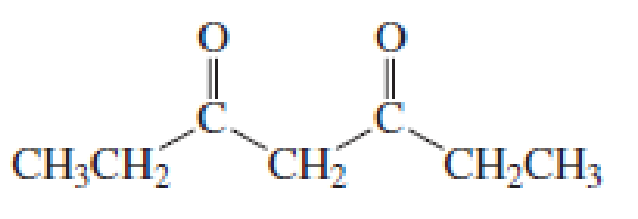
(a)
Interpretation:
The enol tautomer of the given compound has to be drawn and more stable structure has to be identified.
Concept Introduction:
Tautomerism is the ability of a molecule to exist in more than one chemical form.
Tautomers are formed by the migration of a hydrogen atom, accompanied by the switching of a single and neighboring double bond.
The only difference in keto-enol tautomers is the location of hydrogen and double bond.
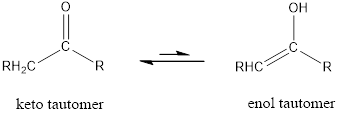
Enol tautomer is much less stable than the keto tautomer.
Enol tautomer is more stable when enol tautomer is aromatic or when the double bonds are conjugated.
Resonance is an electron displacement effect for stabilizing a molecule through delocalization of bonding electrons in the pi orbital.
Delocalized electrons stabilize a compound. The extra stability gains from having delocalized electrons are called resonance stabilization or resonance energy.
Explanation of Solution
Given keto tautomer is,
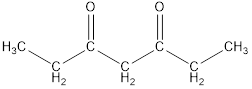
The only difference in keto-enol tautomer is the location of hydrogen and double bond.
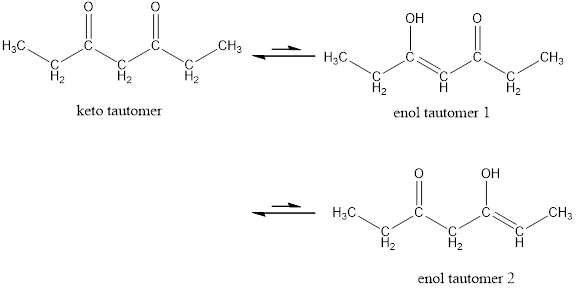
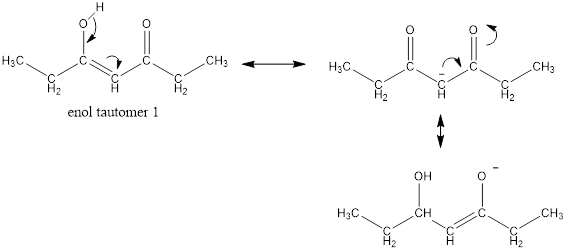
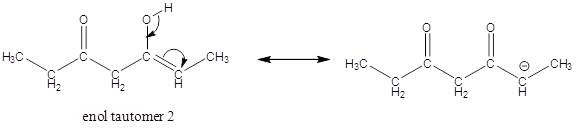
Enol tautomer 1 is more stable than enol tautomer 2.
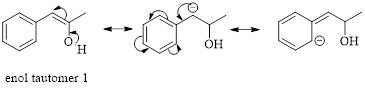
Enol tautomer 1 can undergo delocalization and are more stable.
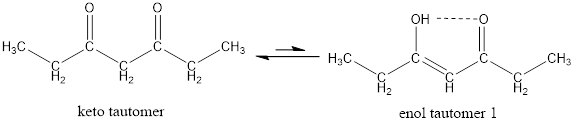
Thus enol tautomer 1 is more stable since it has more resonance structures and also possess intramolecular hydrogen bonding.
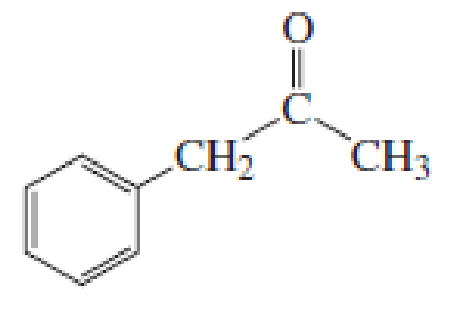
(b)
Interpretation:
The enol tautomer of the given compound has to be drawn and more stable structure has to be identified.
Concept Introduction:
Tautomerism is the ability of a molecule to exist in more than one chemical form.
Tautomers are formed by the migration of a hydrogen atom, accompanied by the switching of a single and neighboring double bond.
The only difference in keto-enol tautomers is the location of hydrogen and double bond.

Enol tautomer is much less stable than the keto tautomer.
Enol tautomer is more stable when enol tautomer is aromatic or when the double bonds are conjugated.
Resonance is an electron displacement effect for stabilizing a molecule through delocalization of bonding electrons in the pi orbital.
Delocalized electrons stabilize a compound. The extra stability gains from having delocalized electrons are called resonance stabilization or resonance energy.
Explanation of Solution
Given keto tautomer is,
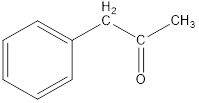
The only difference in keto-enol tautomers is the location of hydrogen and double bond.
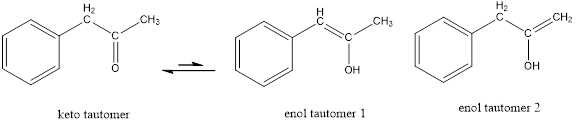
These tautomers undergo resonance and are shown below,
Enol tautomer 1 can undergo delocalization. Enol tautomer 1 is more stable than enol tautomer 2.
The enol tautomer 1 is more stable because there is a conjugation between the double bond and benzene ring. No such conjugation is possible in the enol tautomer 2.
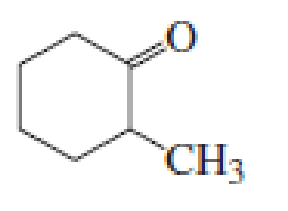
(c)
Interpretation:
The enol tautomer of the given compound has to be drawn and more stable structure has to be identified.
Concept Introduction:
Tautomerism is the ability of a molecule to exist in more than one chemical form.
Tautomers are formed by the migration of a hydrogen atom, accompanied by the switching of a single and neighboring double bond.
The only difference in keto-enol tautomers is the location of hydrogen and double bond.

Enol tautomer is much less stable than the keto tautomer.
Enol tautomer is more stable when enol tautomer is aromatic or when the double bonds are conjugated.
Explanation of Solution
Given keto tautomer is,
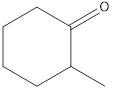
The only difference in keto-enol tautomers is the location of hydrogen and double bond.
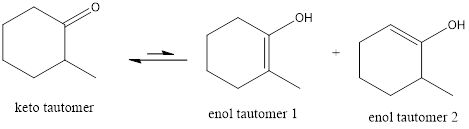

The alkene double bond formed by tautomer 1 is tetra-substituted which is stable than tautomer 2.
Hence, enol tautomer 1 is more stable.
Want to see more full solutions like this?
Chapter 13 Solutions
Pearson eText for Essential Organic Chemistry -- Instant Access (Pearson+)
- Draw all formal charges on the structures below as is and draw 1 resonance structure that is more stable.arrow_forwardPart II. xiao isolated a compound TAD (Ca H 10 N₂) from tobacco and obtained its IR spectrum. Xiao proposed a chemical structure shown below: % Transmittance 4000 3500 3000 2500 2000 Wavenumber (cm-1) 1500 1000 (a) Explain why her proposed structure is inconsistent with the IR spectrum obtained (b) TAD exists as a tautomer of the structure xiao proposed. Draw the structure and explain why it is more compatible with the obtained spectrum. (C) what is the possible source for the fairly intense signal at 1621cm1arrow_forwardAE>AE₁ (Y/N) AE=AE₁ (Y/N) AEarrow_forwardTreatment of 2-phenylpropan-2-amine with methyl 2,4-dibromobutanoate in the presence of a nonnucleophilic base, R3N, involves two successive SN2 reactions and gives compound A. ? NH2 Br Br Propose a structural formula for compound A. You do not have to explicitly draw H atoms. You do not have to consider stereochemistry. In cases where there is more than one answer, just draw one. R3N C14H19NO2 + 2 R3NH*Br Aarrow_forwardCorrectly name this compound using the IUPAC naming system by sorting the components into the correct order. Br IN Ν Harrow_forwardHow is the radical intermediate for this structure formed? Can you please draw arrows from the first radical to the resonance form that would result in this product? I'm lost.arrow_forwardPart VI. (a) calculate the λ max of the compound using woodward - Fieser rules. (b) what types of electronic transitions are present in the compound? (c) what are the prominent peaks in the IR spectrum of the compound?arrow_forwardDon't used Ai solutionarrow_forwardPlease correct answer and don't used hand raitingarrow_forward↑ 0 Quiz List - RCC430M_RU05 X Aktiv Learning App × Qdraw resonance structure ×Q draw resonance structure xb My Questions | bartleby ×+ https://app.aktiv.com Draw a resonance structure of pyrrole that has the same number of pi bonds as the original structure. Include all lone pairs in your structure. + N H a 5 19°F Cloudy Q Search Problem 12 of 15 Atoms, Bonds and Rings Charges and Lone Pairs myhp हजु Undo Reset Remove Done Submit Drag To Pan 2:15 PM 1/25/2025arrow_forwardDon't used hand raitingarrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forwardarrow_back_iosSEE MORE QUESTIONSarrow_forward_ios
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
