
Concept explainers
(a)
Interpretation:
The product of the given reaction is to be predicted.
Concept Introduction:
Addition reaction: A general reaction type in which a substance X-Y adds to the multiple bond of an unsaturated reactant to yield a saturated product that has only single bonds.
Hydrogenation (Addition of
b)
Interpretation:
The products of the reaction to be predicted
Concept Introduction:
Reaction of
Nitration: The substitution of a nitro group for hydrogen on an aromatic ring.
(c)
Interpretation:
The product of the given reaction to be predicted
Concept Introduction:
Addition reaction: A general reaction type in which a substance X-Y adds to the multiple bond of an unsaturated reactant to yield a saturated product that has only single bonds.
Hydration:
When alkene is undergoes hydration with water in the presence of sulfuric acid which yields the alcohol. In this reaction, the water molecule will behave like a hydrogen halide to the alkene which gives the addition product this reaction is known as a hydration reaction.
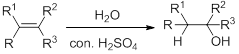
Alkene is reaction with water in the presence of sulfuric acid, first step is proton (
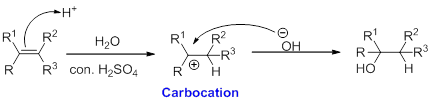
In hydration reaction, sulfuric acid is act as a proton donor, which is the driving force of the reaction. Hydration reaction will not go without acid (sulfuric acid).
Markovnikov’s rule: In the addition of
(d)
Interpretation:
The product of the given reaction to be predicted
Concept Introduction:
Addition reaction: A general reaction type in which a substance X-Y adds to the multiple bond of an unsaturated reactant to yield a saturated product that has only single bonds.
Hydration:
When alkene is undergoes hydration with water in the presence of sulfuric acid which yields the alcohol. In this reaction, the water molecule will behave like a hydrogen halide to the alkene which gives the addition product this reaction is known as a hydration reaction.
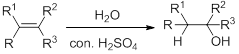
Alkene is reaction with water in the presence of sulfuric acid, first step is proton (

In hydration reaction, sulfuric acid is act as a proton donor, which is the driving force of the reaction. Hydration reaction will not go without acid (sulfuric acid).
Markovnikov’s rule: In the addition of
(e)
Interpretation:
The product of the given reaction to be predicted
Concept Introduction:
Addition reaction: A general reaction type in which a substance X-Y adds to the multiple bond of an unsaturated reactant to yield a saturated product that has only single bonds.
Hydrogenation (Addition of
Want to see the full answer?
Check out a sample textbook solution
Chapter 13 Solutions
FUND.OF GEN CHEM CHAP 1-13 W/ACCESS
- In a cell free preparation of beta-lactamase, penicillin is hydrolyzed in a D2O enriched assay. After one round of catalysis, where would you anticipate finding Deuterium? please help thank youarrow_forwardTo map the active site of -lactamase, the enzyme was hydrolyzed with trypsin to yield a hexapeptide (P1) with the following amino acids. Glu, Lys, Leu, Phe, Met, and Ser. Treatment of P1 with phenyl isothiocyanate yielded a PTH derivative of phenylalanine and a peptide (P2). Treatment of P1 with cyanogenbromide gave an acidic tetrapeptide (P3) and a dipeptide (P4).Treatment of P2 with 1-fluoro-2,4-dinitrobenzene, followed by complete hydrolysis, yields N-2,4-dinitrophenyl-Glu. P1, P2, and P3 contain the active site serine. question: the b-lactamase hydrolyzes the lactam-ring in antibiotics like penicillin. Describe the mechanism, of hydrolysis, insuring to include the involvement of S, D, and K in the reaction sequence. Please help!arrow_forwardThree of these amino acids participate in the proteolytic hydrolysis of polypeptides. Show the charge-relay network generated by the serine proteases and identify the nucleophilic species that initiates the hydrolysis. please help!arrow_forward
- You have isolated a protein and determined that the native molecular weight of the holoenzyme is 160 kD using size exclusion chromatography. Analysis of this protein using SDS-PAGE revealed 2 bands, one at 100 kD and one at 30 kD. 1. Describe the architecture of the polypeptide component of this enzyme. 2. The enzyme was found to be 0.829% NAD (by weight). What further can be said regarding the architecture? can you please help me with question number 2arrow_forwardTo map the active site of -lactamase, the enzyme was hydrolyzed with trypsin to yield a hexapeptide (P1) with the following amino acids. Glu, Lys, Leu, Phe, Met, and Ser. Treatment of P1 with phenyl isothiocyanate yielded a PTH derivative of phenylalanine and a peptide (P2). Treatment of P1 with cyanogenbromide gave an acidic tetrapeptide (P3) and a dipeptide (P4).Treatment of P2 with 1-fluoro-2,4-dinitrobenzene, followed by complete hydrolysis, yields N-2,4-dinitrophenyl-Glu. P1, P2, and P3 contain the active site serine. Question: although S, K, and D are involved in the catalysis, the E in this hexapeptide does not participate in the hydrolysis of the b-lactam ring. Why is that?arrow_forwardTo map the active site of beta-lactamase, the enzyme was hydrolyzed with trypsin to yield a hexapeptide (P1) with the following amino acids. Glu, Lys, Leu, Phe, Met, and Ser. a) Using the experimental results described below deduce the primary sequence of the active site hexapeptide. Treatment of P1 with phenyl isothiocyanate yielded a PTH derivative of phenylalanine and a peptide (P2). Treatment of P1 with cyanogenbromide gave an acidic tetrapeptide (P3) and a dipeptide (P4).Treatment of P2 with 1-fluoro-2,4-dinitrobenzene, followed by complete hydrolysis, yields N-2,4-dinitrophenyl-Glu. P1, P2, and P3 contain the active site serine. please help!arrow_forward
- The beta-lactamase hydrolyzes the lactam-ring in penicillin. Describe the mechanism of hydrolysis, insuring to include the involvement of S, D, & K in the reaction sequence. Please helparrow_forwardTo map the active site of beta-lactamase, the enzyme was hydrolyzed with trypsin to yield a hexapeptide (P1) with the following amino acids. Glu, Lys, Leu, Phe, Met, and Ser. Treatment of P1 with phenyl isothiocyanate yielded a PTH derivative of phenylalanine and a peptide (P2). Treatment of P1 with cyanogenbromide gave an acidic tetrapeptide (P3) and a dipeptide (P4).Treatment of P2 with 1-fluoro-2,4-dinitrobenzene, followed by complete hydrolysis, yields N-2,4-dinitrophenyl-Glu. P1, P2, and P3 contain the active site serine. Why doesn't D in this hexapeptide not participate in the hydrolysis of the beta-lactam ring even though S, K, and D are involved in the catalyst?arrow_forwardTo map the active site of -lactamase, the enzyme was hydrolyzed with trypsin to yield a hexapeptide (P1) with the following amino acids. Glu, Lys, Leu, Phe, Met, and Ser. Treatment of P1 with phenyl isothiocyanate yielded a PTH derivative of phenylalanine and a peptide (P2). Treatment of P1 with cyanogenbromide gave an acidic tetrapeptide (P3) and a dipeptide (P4).Treatment of P2 with 1-fluoro-2,4-dinitrobenzene, followed by complete hydrolysis, yields N-2,4-dinitrophenyl-Glu. P1, P2, and P3 contain the active site serine. Using the experimental results described above derive the primary sequence of the active site hexapeptide. Please help!arrow_forward
- Which type of enzyme catalyses the following reaction? oxidoreductase, transferase, hydrolase, lyase, isomerase, or ligase.arrow_forward+NH+ CO₂ +P H₂N + ATP H₂N NH₂ +ADParrow_forwardWhich type of enzyme catalyses the following reaction? oxidoreductase, transferase, hydrolase, lyase, isomerase, or ligase.arrow_forward
 Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning
Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning
Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax





