
Concept explainers
(a)
Interpretation:
Chemical equation that shows reactants, product and catalyst needed for the reaction of ethene with water has to be written.
Concept Introduction:
In this reaction no atoms or group of atoms are removed. Instead the unsaturated bond is reduced to saturated bond. A general scheme for addition reaction of
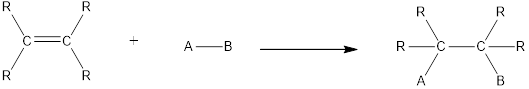
Hydration is an example of addition reaction. In this reaction, a water molecule is incorporated into the molecules of organic compound. Hydration of alkene results in the formation of alcohol, where one carbon atom gets hydrogen atom added and the other carbon atom gets hydroxyl group added to it. This reaction requires a small amount of sulphuric acid as catalyst.
(b)
Interpretation:
Chemical equation that shows reactants, product and catalyst needed for the reaction of ethene with bromine has to be written.
Concept Introduction:
Chemical reaction in which an atom or a group of atoms are added to each carbon atom of a carbon‑carbon multiple bond in a hydrocarbon or hydrocarbon derivative is known as addition reaction.
In this reaction no atoms or group of atoms are removed. Instead the unsaturated bond is reduced to saturated bond. A general scheme for addition reaction of alkene can be given as shown below,

Halogenation reaction is an example of addition reaction. In this reaction, the halogen atoms are added across the double bonds. Chlorination and bromination are the most commonly used halogenation reaction. For halogenation reaction, no catalyst is required.
(c)
Interpretation:
Chemical equation that shows reactants, product and catalyst needed for the reaction of ethene with hydrogen iodide has to be written.
Concept Introduction:
Chemical reaction in which an atom or a group of atoms are added to each carbon atom of a carbon‑carbon multiple bond in a hydrocarbon or hydrocarbon derivative is known as addition reaction.
In this reaction no atoms or group of atoms are removed. Instead the unsaturated bond is reduced to saturated bond. A general scheme for addition reaction of alkene can be given as shown below,

Asymmetrical addition reaction is the one in which two different atoms or group of atoms are substituted across the multiple bond resulting in the formation of product. No catalyst is required for this reaction.
(d)
Interpretation:
Chemical equation that shows reactants, product and catalyst needed for the reaction of ethene with iodine has to be written.
Concept Introduction:
Chemical reaction in which an atom or a group of atoms are added to each carbon atom of a carbon‑carbon multiple bond in a hydrocarbon or hydrocarbon derivative is known as addition reaction.
In this reaction no atoms or group of atoms are removed. Instead the unsaturated bond is reduced to saturated bond. A general scheme for addition reaction of alkene can be given as shown below,

Halogenation reaction is an example of addition reaction. In this reaction, the halogen atoms are added across the double bonds. Chlorination and bromination are the most commonly used halogenation reaction. For halogenation reaction, no catalyst is required.
Want to see the full answer?
Check out a sample textbook solution
Chapter 13 Solutions
EBK GENERAL, ORGANIC, AND BIOLOGICAL CH
- please draw in the answers, thank youarrow_forwarda. On this first grid, assume that the DNA and RNA templates are read left to right. DNA DNA mRNA codon tRNA anticodon polypeptide _strand strand C с A T G A U G C A TRP b. Now do this AGAIN assuming that the DNA and RNA templates are read right to left. DNA DNA strand strand C mRNA codon tRNA anticodon polypeptide 0 A T G A U G с A TRParrow_forwardplease answer all question below with the following answer choice, thank you!arrow_forward
- please draw in the answeres, thank youarrow_forwardA) What is being shown here?B) What is indicated by the RED arrow?C) What is indicated by the BLUE arrow?arrow_forwardPlease identify the curve shown below. What does this curve represent? Please identify A, B, C, D, and E (the orange oval). What is occurring in these regions?arrow_forward
- Please identify the test shown here. 1) What is the test? 2) What does the test indicate? How is it performed? What is CX? 3) Why might the test be performed in a clinical setting? GEN CZ CX CPZ PTZ CACarrow_forwardDetermine how much ATP would a cell produce when using fermentation of a 50 mM glucose solution?arrow_forwardDetermine how much ATP would a cell produce when using aerobic respiration of a 7 mM glucose solution?arrow_forward
- Determine how much ATP would a cell produce when using aerobic respiration to degrade one small protein molecule into 12 molecules of malic acid, how many ATP would that cell make? Malic acid is an intermediate in the Krebs cycle. Assume there is no other carbon source and no acetyl-CoA.arrow_forwardIdentify each of the major endocrine glandsarrow_forwardCome up with a few questions and answers for umbrella species, keystone species, redunant species, and aquatic keystone speciesarrow_forward
- Essentials of Pharmacology for Health ProfessionsNursingISBN:9781305441620Author:WOODROWPublisher:Cengage
 Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College
Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage Learning
Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage Learning





